| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com |
Case Report
Volume 17, Number 3, March 2026, pages 121-127
Hemobilia as a Complication of Transjugular Liver Biopsy Causing Acute Pancreatitis and Obstructive Jaundice: A Case Report and Minireview
Usama Sakhawata, e, Ahmed Shehadaha, Behrawar Ahmadb, Atif Nawaz Malikc, Umar Hayatd, Ali Marhabaa
aDepartment of Gastroenterology, United Health Services Hospitals, Binghamton, NY, USA
bDepartment of Internal Medicine, United Health Services Hospitals, Binghamton, NY, USA
cDepartment of Gastroenterology, Sheikh Zayed Medical College and Hospital, Rahim Yar Khan, Pakistan
dDepartment of Internal Medicine, Geisinger Wyoming Valley Medical Center, Wilkes Barre, PA, USA
eCorresponding Author: Usama Sakhawat, Department of Gastroenterology, United Health Services Hospitals, Binghamton, NY, USA
Manuscript submitted December 22, 2025, accepted January 22, 2026, published online February 2, 2026
Short title: TJLB Hemobilia: Pancreatitis & Obstructive Jaundice
doi: https://doi.org/10.14740/jmc5269
| Abstract | ▴Top |
Liver biopsy is a critical diagnostic tool in the evaluation of certain hepatic and biliary disorders, particularly when non-invasive testing is inconclusive. It allows for definitive histopathologic assessment, guides prognostication, and informs therapeutic decision-making. Several techniques for liver biopsy are available, including percutaneous, transjugular, and surgical approaches. Among the available techniques, transjugular liver biopsy (TJLB) is considered to have a superior safety profile compared to other techniques. However, complications still occur, and hemobilia is one such rare but potentially serious event. While multiple cases of hemobilia following percutaneous liver biopsy have been reported, occurrences after TJLB are exceedingly uncommon. We present a case of hemobilia developing after a TJLB. The diagnosis can be challenging because the initial presentation is often nonspecific. In our case, the patient did not exhibit overt gastrointestinal bleeding but presented with acute pancreatitis and obstructive jaundice, which led to further evaluation and the eventual diagnosis of hemobilia. Endoscopic retrograde cholangiopancreatography (ERCP) was both diagnostic and therapeutic, resulting in complete clinical and biochemical recovery. This case highlights the importance of maintaining a high index of suspicion for hemobilia following TJLB, particularly in patients presenting with unexplained jaundice or pancreatitis even in the absence of overt gastrointestinal bleeding.
Keywords: Endoscopy; Biliary disease; Pancreatic disease
| Introduction | ▴Top |
Transjugular liver biopsy (TJLB) is generally considered safer and associated with a lower rate of adverse events compared to the percutaneous approach. Nevertheless, complications may still occur. Hemobilia is a rare but potentially serious adverse event, with an estimated incidence of less than 0.1% among all TJLB procedures [1–3]. Other recognized causes of hemobilia include invasive hepatobiliary interventions, choledocholithiasis, and hepatobiliary malignancies.
The clinical presentation is often nonspecific, and only a minority of patients exhibit the classical Quincke’s triad of upper gastrointestinal (GI) bleeding, jaundice, and right upper quadrant pain. Diagnosis typically relies on a combination of clinical assessment, imaging studies, angiography, and endoscopic evaluation. Management is multifaceted—while most cases are self-limited, severe bleeding may necessitate transcatheter angiographic embolization or, rarely, surgical intervention. Relief of biliary obstruction often requires endoscopic retrograde cholangiopancreatography (ERCP).
We present a rare case of hemobilia following TJLB, in which the patient developed acute pancreatitis and obstructive jaundice. The diagnosis was confirmed on ERCP, which proved to be both diagnostic and therapeutic.
| Case Report | ▴Top |
A 49-year-old male with medical history of asthma, diabetes mellitus type II, hypertension, hyperlipidemia, atrial fibrillation on apixaban, Wolff-Parkinson-White syndrome, obstructive sleep apnea, and gastro-esophageal reflux disease was found to have abnormal liver function tests (LFTs) on an annual physical exam and lab work. The patient at this time was asymptomatic with no abdominal or other systemic symptoms. LFTs - aspartate aminotransferase (AST) 71 U/L (normal < 59), alanine aminotransferase (ALT) 124 U/L (normal < 50), total bilirubin 0.5 mg/dL (normal < 1.3), and alkaline phosphatase (ALP) 37 U/L (normal 38–126). To delineate the cause of abnormal LFTs, he underwent further testing. Hepatitis B surface antigen, hepatitis B core antibody, hepatitis C Virus antibody, anti-tissue transglutaminase IgA, anti-mitochondrial antibody, and anti-nuclear antibody were negative. Ceruloplasmin levels were normal. Anti-smooth muscle antibody (ASMA) level was positive at 1:40. Since the ASMA was positive, autoimmune hepatitis was suspected.
An ultrasound (US) of the abdomen was done, which revealed a fatty liver, gallbladder sludge, and a dilated common bile duct measuring 9.6 mm. For evaluation of the dilated common bile duct, a magnetic resonance cholangiopancreatography (MRCP) was done. This showed stable dilation of the common bile duct without evidence of filling defect or stricture and hepatic steatosis.
Since autoimmune hepatitis was suspected, the patient underwent a TJLB. Apixaban was held for 2 days prior to the procedure. The procedure was performed successfully without any intra-procedural complications. Hepatic venous pressure gradient (HVPG) was measured, which was normal, indicating no portal hypertension. Hepatic parenchymal tissue was obtained for histopathological examination. Pathology findings were consistent with fatty liver disease, possibly metabolic-associated steatohepatitis (MASH), with a non-alcoholic fatty liver disease (NAFLD) score of 3/8 (steatosis 1/3 noted in Fig. 1a, d, balloon degeneration 1/2 noted in Fig. 1b, c, and lobular inflammation 1/3 noted in Fig. 1d), mild pericellular and periportal fibrosis stage 1/4 (NASH clinical research network fibrosis staging system).
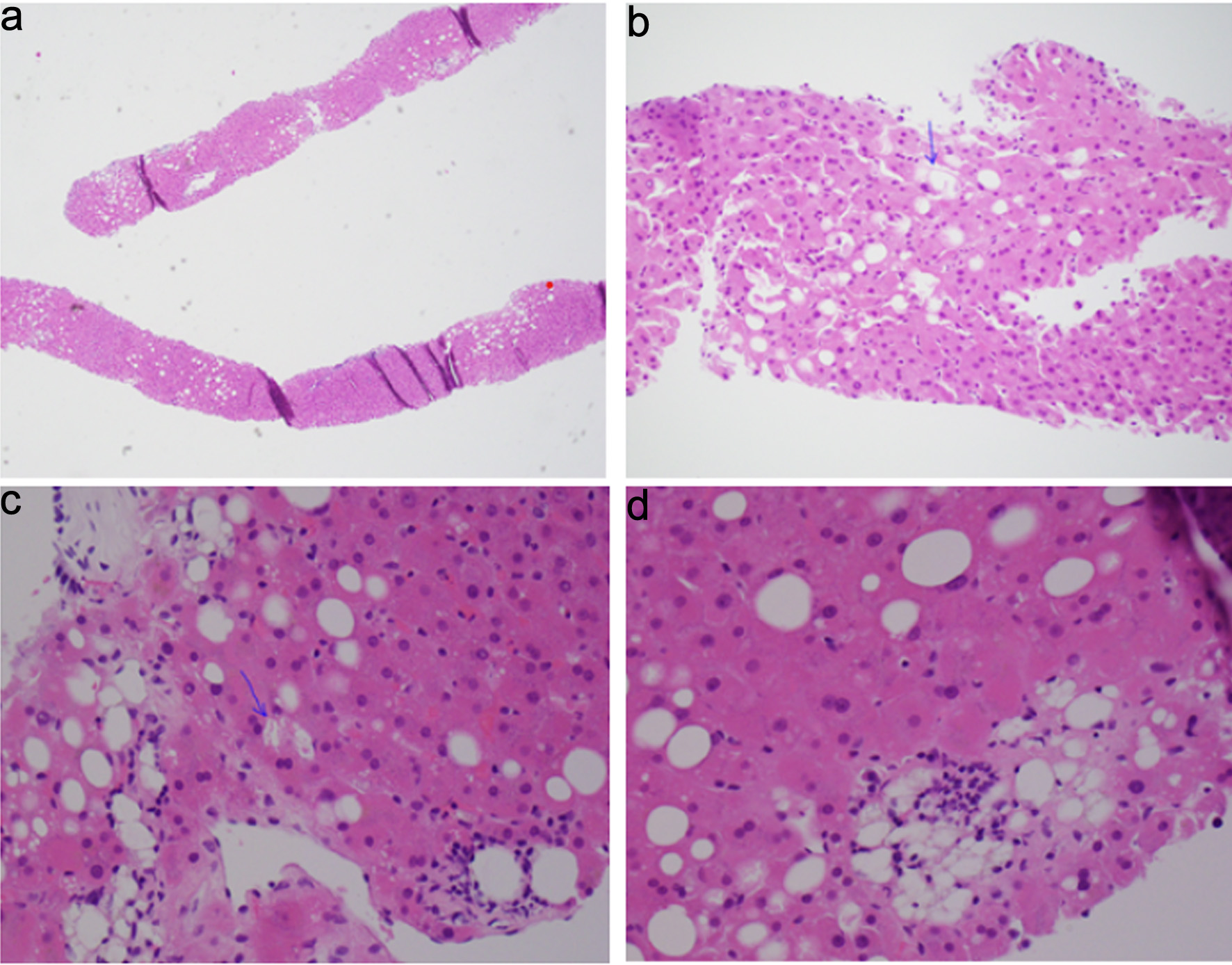 Click for large image | Figure 1. Histopathology examination of hepatic parenchyma. (a) Steatosis, involving 10–15% of hepatocytes. (b, c) Ballooning of hepatocytes (arrows). (d) Steatosis and lobular inflammation. |
Apixaban was restarted 1 day after the procedure. Two days after the procedure, the patient presented to the emergency room with severe epigastric abdominal pain radiating to the back, along with nausea and vomiting. He denied hematemesis, hematochezia, or melena. He denied any fevers, rigors, or chills. He was hemodynamically stable with normal vitals. Lab workup was significant for abnormal LFTs—AST elevated to 113 U/L, ALT elevated to 97 U/L, total bilirubin 0.5 mg/dL, and alkaline phosphatase 48 U/L. Lipase was elevated at > 4,000 International Units/L (normal < 300). Complete blood count (CBC) revealed mild leukocytosis with a white blood cell count (WBC) of 10.9 (normal range 4–10.5 × 103/µL), hemoglobin (Hb) of 13.5 g/dL (normal range 13–18 g/dL), and platelets 322 (normal range 125–425 × 103/µL). Prothrombin time was slightly above normal at 13.1 s (normal 9–13 s), and partial thromboplastin time was normal at 34 s (22–37 s).
Computed tomography (CT) scan of the abdomen and pelvis showed hepatic steatosis but no acute intra-abdominal pathology. The patient was admitted to the hospital for management of acute pancreatitis.
The patient was initiated on supportive management with intravenous normal saline, analgesics, and antiemetics. The following day, there was a significant rise in LFTs. AST increased from 113 to 353 IU/L, ALT from 97 to 282 IU/L, and total bilirubin from 0.5 to 2.4 mg/dL, while alkaline phosphatase remained within normal limits. An acute decline in hemoglobin was also observed, decreasing from 13.5 to 10.6 g/dL.
At this point, the working diagnosis was acute pancreatitis of unclear etiology. On the subsequent day, LFTs continued to worsen, with AST increasing from 353 to 601 IU/L, ALT from 282 to 423 IU/L, and total bilirubin from 2.4 to 4.4 mg/dL. Alkaline phosphatase remained normal at 111 IU/L.
General surgery and gastroenterology services were consulted. In view of the recent liver biopsy and subsequent development of abnormal LFTs, obstructive jaundice, and acute anemia, hemobilia was suspected. As part of the diagnostic workup, a hepatobiliary iminodiacetic acid (HIDA) scan was performed, which demonstrated normal radiotracer uptake with no evidence of obstruction. However, MRCP revealed an elongated filling defect in the distal common bile duct with associated intrahepatic and extrahepatic ductal dilatation, findings consistent with biliary obstruction (Fig. 2a, b).
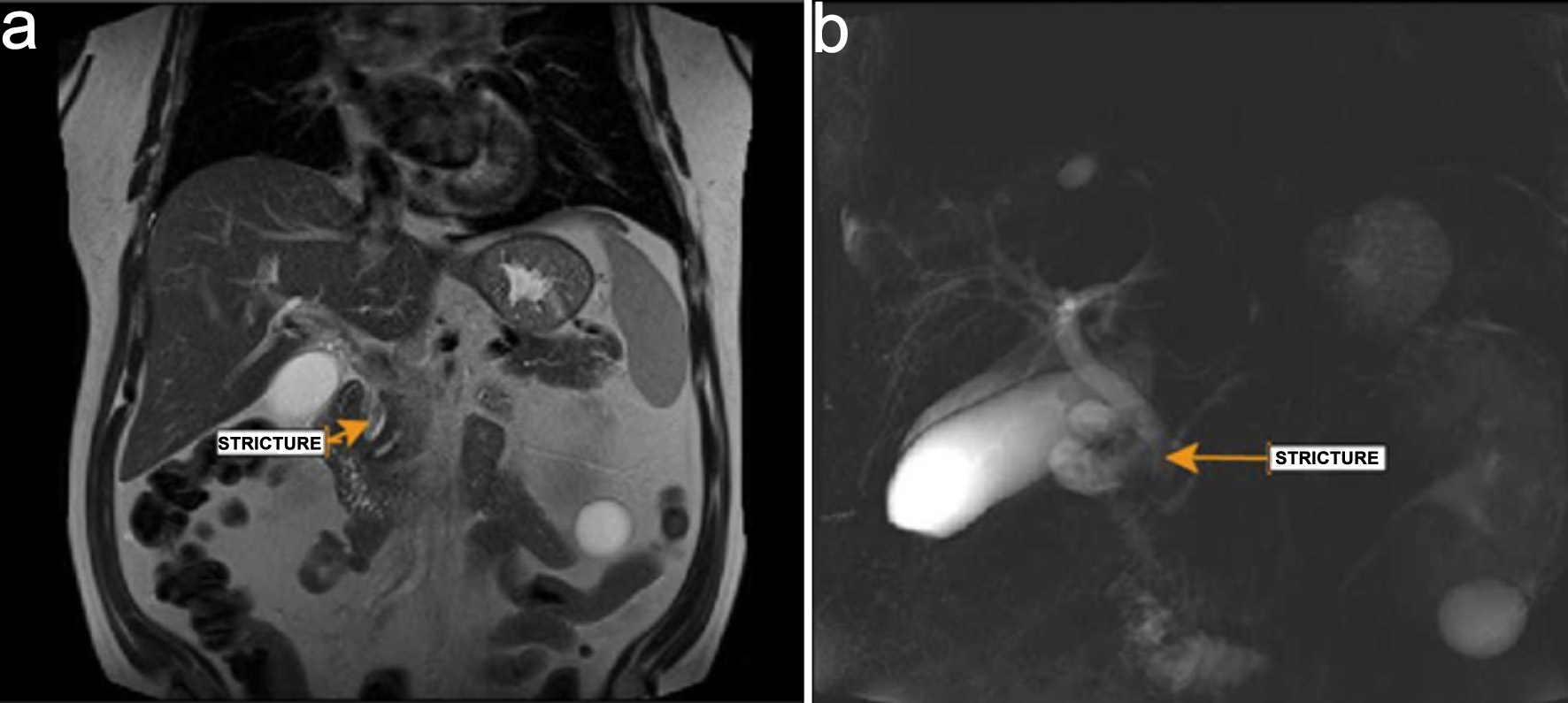 Click for large image | Figure 2. Magnetic resonance cholangiopancreatography. (a) Stricture (arrow) noted in common bile duct. (b) Dilated bile duct with distal stricture (arrow). |
An emergent ERCP was performed. The major papilla appeared small, and the pancreatic duct was normal. The cholangiogram demonstrated a dilated biliary tree with multiple filling defects (Fig. 3a, b). A biliary sphincterotomy was performed, and the bile ducts were swept, yielding blood clots that were subsequently removed. A prophylactic pancreatic duct stent was placed to prevent post-ERCP pancreatitis. A temporary plastic stent was placed in the common bile duct to facilitate drainage (Fig. 4a, b).
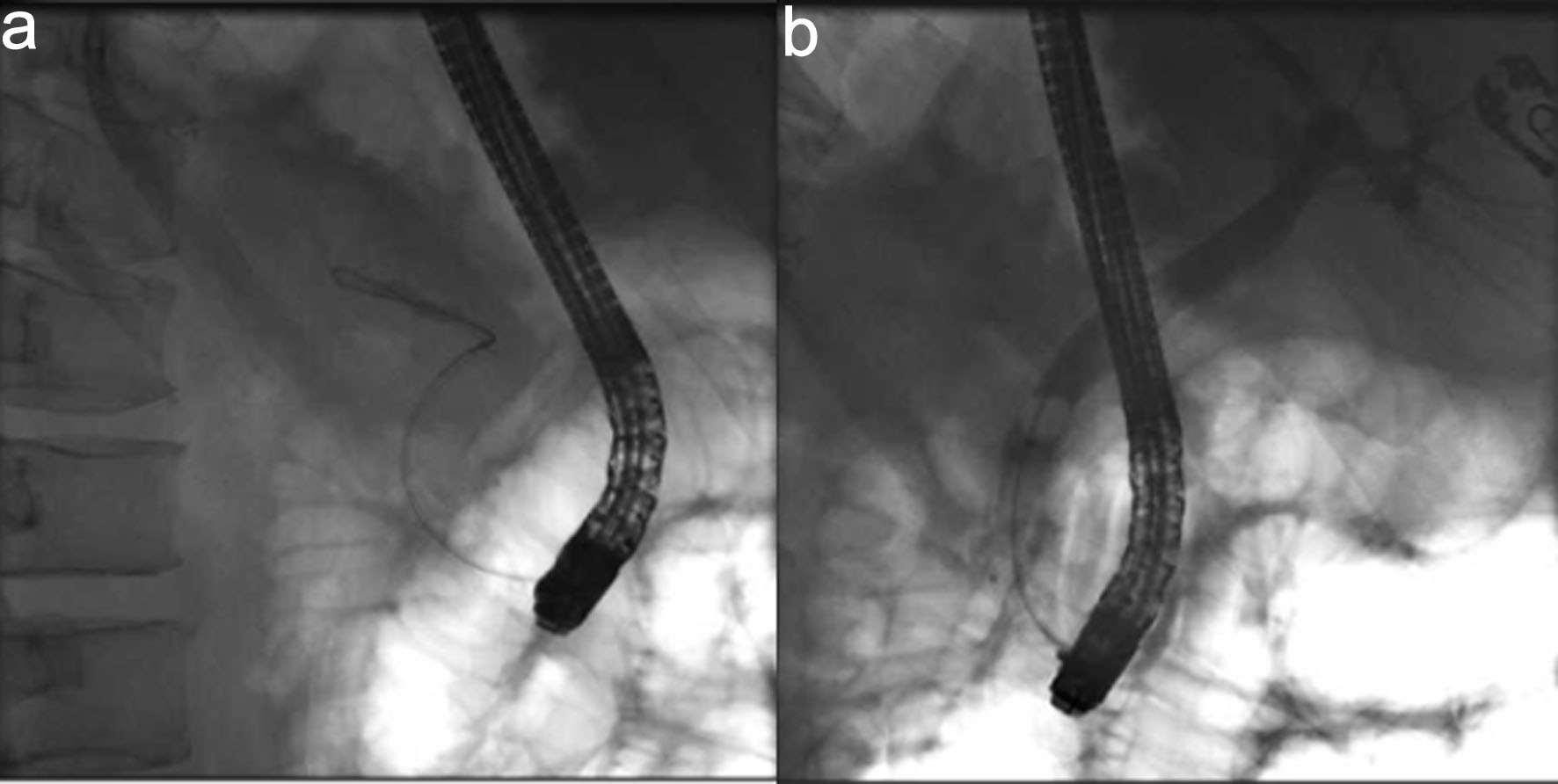 Click for large image | Figure 3. Cholangiogram. (a) Fluroscopic image revealing wire cannulation of bile duct. (b) Cholangiogram revealing a dilated biliary tree with filing defects. |
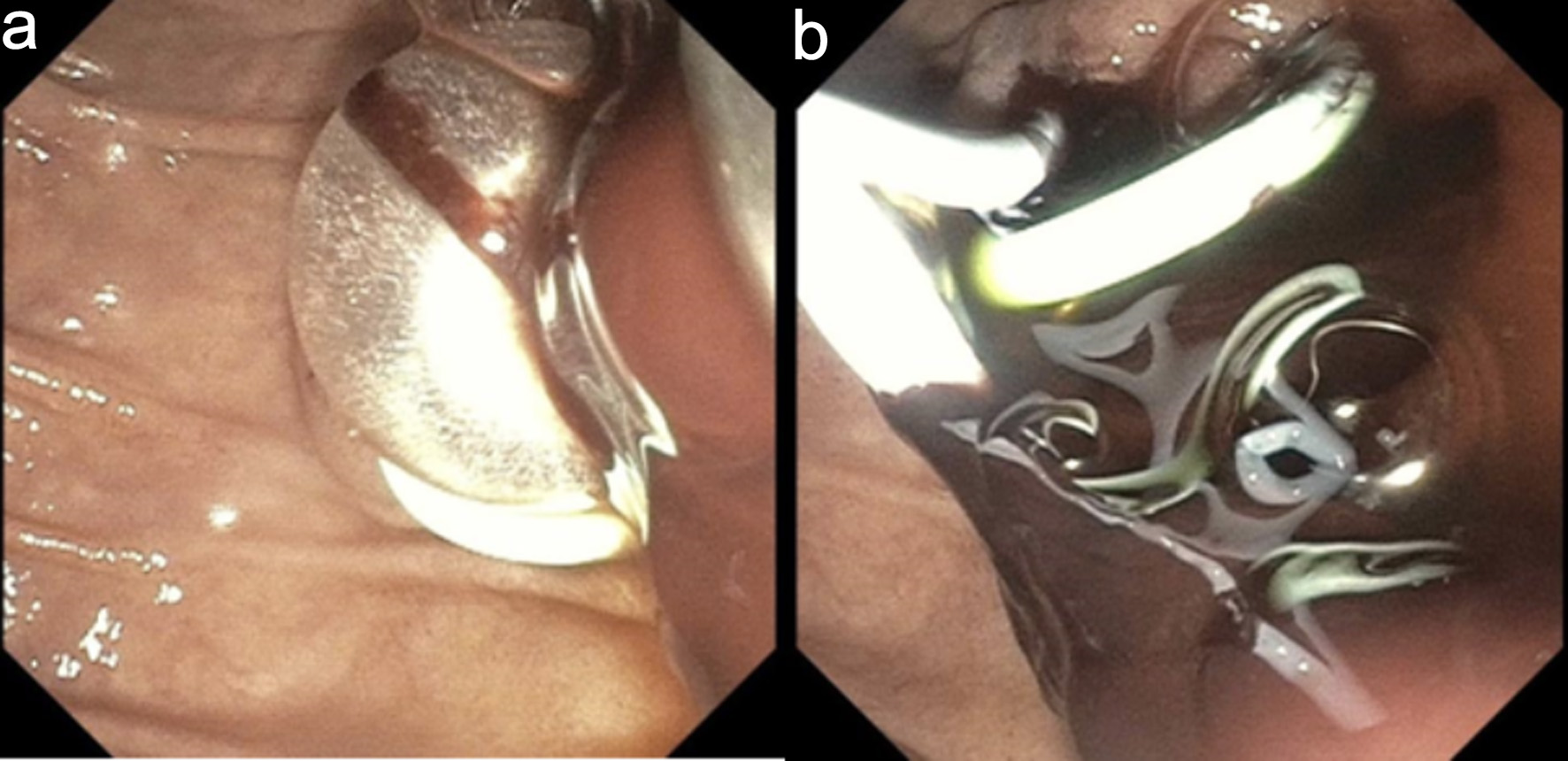 Click for large image | Figure 4. Endoscopic retrograde cholangiopancreatography. (a) Endoscopic sphincterotomy followed by balloon sweep and extraction of blood clots. (b) Plastic bile duct and pancreatic duct stents were deployed to maintain biliary drainage and to prevent post-ERCP pancreatitis, respectively. |
These findings confirmed hemobilia as the underlying cause of acute pancreatitis and obstructive jaundice. Following the procedure, there was complete resolution of obstructive jaundice with normalization of LFTs, and the patient recovered fully. Apixaban remained on hold at that time.
| Discussion | ▴Top |
In this case report, we specifically describe a rare presentation of hemobilia following TJLB, complicated by obstructive jaundice and acute pancreatitis in the absence of overt GI bleeding. The diagnosis was delayed due to the lack of classic bleeding manifestations and was ultimately established by ERCP, which revealed blood clots obstructing the distal bile duct. This case highlights an uncommon but clinically important complication of a procedure generally regarded as low risk.
The focus of this discussion is the epidemiology, pathophysiology, clinical presentation, complications, and management of hemobilia.
Epidemiology, procedural risks, and the pathophysiology
The reported incidence of significant hemobilia following all types of liver biopsy procedures is less than 0.1% [1, 2]. Theoretically, TJLB carries a lower risk of hemobilia than percutaneous biopsy. A large multicenter retrospective study by Mammen et al supported this fact, as only four cases out of 601 patients (0.67%) developed hemobilia after TJLB [3]. There have been cases of hemobilia reported following transjugular intrahepatic portosystemic shunt placement [4, 5]. Other causes of hemobilia include, but are not limited to, hepatobiliary surgery, endoscopic hepatobiliary procedures, accidental hepatic trauma, hepatocellular carcinoma, cystic artery pseudoaneurysm, severe choledocholithiasis, and spontaneous hemobilia in the setting of anticoagulation [6].
TJLB is preferred over other liver biopsy methods [7], because it minimizes the risk of free intraperitoneal hemorrhage by containing any bleeding within the vascular system and is considered more reliable in patients with coagulopathy, significant ascites, or those requiring hemodynamic measurements.
However, complications can still occur. The intrahepatic bile ducts and hepatic vasculature lie in close proximity; therefore, multiple needle passes through the liver during TJLB may injure vascular structures and lead to the formation of iatrogenic fistulae or vasculobiliary communications. These are usually arterio-biliary in nature, although veno-biliary fistulae can also occur. This mechanism underlies the pathophysiology of hemobilia and bleeding into the biliary tree [6]. In some cases, a pseudoaneurysm may develop due to procedural injury to blood vessels, which may subsequently communicate with the bile duct and form a fistula [8].
Bleeding into the biliary tree can lead to formation of clots that can cause ductal blockage, leading to obstructive jaundice [9]. Distal bile duct obstruction functionally blocks the pancreatico-biliary channel, resulting in pancreatic juice stasis. This leads to activation of pancreatic zymogens and development of acute inflammation, clinically manifesting as acute pancreatitis [10]. Serum lipase may dramatically rise up to 4,000 U/L, as reported in our case. The first ever case of acute pancreatitis due to hemobilia was documented in 1975 following percutaneous trans-hepatic cholangiogram [11].
Clinical presentation
The signs and symptoms of hemobilia usually appear within 24 h to 7 days after the procedure [12]. The presentation can be nonspecific, often leading to a delay in diagnosis. The classic Quincke triad (jaundice, biliary colic, and upper GI bleeding) is present in only 10–25% of cases [13]. In one study, only 13.8% of patients presented with the Quincke triad [6, 14]. When hemobilia occurs, patients typically present with overt GI bleeding manifestations such as hematemesis [15] or melena. In our case, the patient did not exhibit any signs of overt GI bleeding. The diagnosis was only suspected following an acute drop in hemoglobin, without any reasonable alternative explanation for anemia other than hemobilia. There was also a significant elevation in LFTs, with a pattern suggestive of obstructive jaundice. Moreover, the presence of pancreatitis hinted at impaired drainage of the pancreatic duct due to an obstructing clot. Therefore, a high index of suspicion is required in the appropriate clinical setting to consider this diagnosis.
Role of imaging
Cross-sectional imaging, such as CT angiography, is the first-line, non-invasive diagnostic test of choice when there is a strong suspicion of hemobilia. It can detect blood clots within the biliary system, pseudoaneurysms, and other vascular malformations [16]. However, data on the sensitivity and specificity of CT angiography in detecting hemobilia are limited. Therefore, the study may yield false-negative results, particularly in cases without overt GI bleeding.
HIDA scan has a limited role in diagnosing hemobilia. It is primarily designed to detect bile leaks and assess biliary patency. It cannot reliably distinguish between different types of fluid collections (bile, blood, pus, or serous fluid) due to their similar imaging characteristics.
The role of abdominal ultrasonography is limited, as its diagnostic utility is highly operator-dependent [6]. MRCP can also play a useful role in diagnosis; several case reports have described its confirmatory value [17]. It can identify ductal obstruction and help differentiate blood clots from stones or tumors.
Catheter-based angiography remains the gold standard for diagnosis, with a reported sensitivity of over 90%, particularly in patients with ongoing, significant overt GI bleeding manifesting as hematemesis or melena [16, 18]. Angiography can detect arterio-biliary and arterio-portal fistulae, as well as aneurysms [18]. Angiography has its limitations. Its diagnostic yield is reduced in cases of intermittent, low-volume, or self-limited bleeding, and angiographic findings may be negative in the absence of active hemorrhage at the time of the procedure. Additionally, angiography is an invasive modality.
Management
There are two main principles of management: 1) Stabilization and control of bleeding; 2) Relief of biliary obstruction.
Initial management is conservative and includes measures necessary for hemodynamic resuscitation, correction of coagulopathy if any, holding anticoagulants if any (as in our case), correction of anemia with transfusion if necessary. In most cases, the bleeding resolves spontaneously with conservative management without the need for further intervention [14, 19].
Endoscopic evaluation
1) Esophagogastroduodenoscopy (EGD)
EGD can play a pivotal role in diagnosis, as it allows direct visualization of the ampulla of Vater. The diagnostic yield largely depends on the rate and duration of bleeding [16]. The key endoscopic finding in hemobilia is active bleeding or blood oozing from the papilla during ongoing hemorrhage. However, when bleeding is slow or intermittent, blood may clot and cause biliary obstruction, as it does not mix with bile and instead forms separate layers due to differences in gravity and surface tension [18, 20]. In such cases, a blood clot may be visible at the ampulla—or, at times, no visible finding may be present. A side-viewing duodenoscope provides superior visualization of the papilla. Nevertheless, the absence of visible bleeding at the ampulla does not exclude the diagnosis. The diagnostic yield of endoscopy is variable; in one study, only 12% of examinations were diagnostic [19], and in a retrospective analysis, blood oozing from the ampulla of Vater was observed in only 37.5% of patients [14].
In addition, EGD can also identify and rule out other causes of upper GI bleeding, such as peptic ulcer disease or esophageal variceal bleeding.
2) ERCP
ERCP has both diagnostic and therapeutic roles. Cholangiography can identify filling defects within the bile duct, while a variety of therapeutic interventions can be performed. Sphincterotomy and clot extraction relieve biliary obstruction, thereby resolving abdominal pain and jaundice [14]. In cases of uncontrolled hemobilia, a temporary metal stent may be placed for hemostatic purposes [21, 22], and balloon tamponade can also aid in achieving hemostasis. Placement of a plastic or metallic stent after clot extraction allows for continuous biliary drainage, preventing cholestasis-related complications such as cholangitis. It is important to note that bile has a natural fibrinolytic activity [19], which is impaired when bile flow is interrupted, preventing the dissolution of clots. Consequently, stagnant blood clots may persist in the bile duct due to this disruption of the bile’s fibrinolytic function.
Impact of anticoagulation
TJLB is considered a high bleeding-risk procedure. According to Society of Interventional Radiology consensus guidelines, apixaban should be held for four doses in patients with normal renal function (CrCl ≥ 50 mL/min) prior to the procedure and resumed 24 h afterward [23]. In our case, apixaban was held for 48 h before the procedure and resumed 24 h post-procedure, in accordance with these guidelines. Although the recommended intervals for holding and resuming anticoagulation were followed, anticoagulation may still have contributed to the development or exacerbation of hemobilia in the setting of underlying vascular injury.
Transarterial embolization
Transarterial embolization (TAE) is a minimally invasive and effective treatment for hemobilia, with reported success rates of 80–100% [6]. Marynissen et al reported a 100% success rate with TAE without any major complications [24]. The procedure typically involves the use of microcoils or bioparticles. In our case, there was no overt GI bleeding or hemodynamic instability that would necessitate TAE. ERCP with stent placement proved adequate, and no rebleeding was observed.
Surgery
Surgical management is considered when TAE has failed or in the presence of coexisting cholecystitis. Surgical options include ligation of the bleeding vessel and excision of a pseudoaneurysm, if present. In cases of persistent bleeding, resection of the affected liver segment may be necessary [19]. The choice of surgical approach depends on the type and severity of the injury. Nonetheless, TAE remains the first-line therapy [6].
Conclusions
TJLB is generally preferred over the percutaneous approach due to its superior safety profile and lower risk of complications. However, adverse events may still occur, and hemobilia represents one such rare but potentially serious complication. Hemobilia following TJLB often presents atypically, requiring a high index of suspicion in the appropriate clinical context to establish the diagnosis. The diagnosis is established through a combination of clinical assessment, imaging studies, angiography, and endoscopic evaluation. Endoscopic evaluation can serve as both a diagnostic and therapeutic modality, as demonstrated in our case.
Learning points
Hemobilia after TJLB is rare but clinically significant and often presents atypically. Despite the superior safety profile of TJLB compared with percutaneous biopsy, hemobilia can still occur and may present without overt GI bleeding. Unexplained anemia, obstructive-pattern liver enzyme abnormalities, or acute pancreatitis within days of TJLB should prompt consideration of hemobilia.
Hemobilia can cause secondary complications such as obstructive jaundice and acute pancreatitis. Intraluminal blood clots within the biliary tree may obstruct the distal bile duct, impairing biliary and pancreatic drainage. This mechanism can lead to cholestasis and pancreatitis, even in the absence of classic Quincke’s triad, underscoring the need for a high index of suspicion.
ERCP plays a crucial diagnostic and therapeutic role in hemobilia with biliary obstruction. ERCP allows direct visualization of biliary filling defects, facilitates clot extraction, and enables therapeutic interventions such as sphincterotomy and stent placement. In stable patients without ongoing hemorrhage, ERCP alone may be sufficient, obviating the need for angiographic embolization or surgical intervention.
Acknowledgments
This article was previously presented as a meeting abstract at the 2025 ACG Annual Scientific Meeting.
Financial Disclosure
The authors received no financial support for the research, authorship, or publication of this article.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Informed consent has been obtained.
Author Contributions
Usama Sakhawat, primary author, contributed to all parts of the report from collecting data, writing abstract, case presentation, literature review, discussion and finalizing/reviewing the final version. Ahmed Shehadah contributed to literature review and discussion. Behrawar Ahmad contributed to case presentation, literature review and discussion. Atif Nawaz Malik contributed to case literature review, discussion and conclusion. Umar Hayat contributed to discussion and conclusion. Ali Marhaba contributed to case presentation, discussion and conclusion.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2(2):165-173.
doi pubmed - Savader SJ, Trerotola SO, Merine DS, Venbrux AC, Osterman FA. Hemobilia after percutaneous transhepatic biliary drainage: treatment with transcatheter embolotherapy. J Vasc Interv Radiol. 1992;3(2):345-352.
doi pubmed - Mammen T, Keshava SN, Eapen CE, Raghuram L, Moses V, Gopi K, Babu NS, et al. Transjugular liver biopsy: a retrospective analysis of 601 cases. J Vasc Interv Radiol. 2008;19(3):351-358.
doi pubmed - Gonzalez-Abraldes J, Moitinho E, Garcia-Pagan JC, Escorsell A, Salmeron JM, Gilabert R, Real M, et al. Selective arterial embolization for life threatening hemobilia after transjugular intrahepatic portosystemic shunt placement. J Hepatol. 2001;34(1):174-176.
doi pubmed - Rossle M, Siegerstetter V, Huber M, Ochs A. The first decade of the transjugular intrahepatic portosystemic shunt (TIPS): state of the art. Liver. 1998;18(2):73-89.
doi pubmed - Cathcart S, Birk JW, Tadros M, Schuster M. Hemobilia: an uncommon but notable cause of upper gastrointestinal bleeding. J Clin Gastroenterol. 2017;51(9):796-804.
doi pubmed - Sue MJ, Lee EW, Saab S, McWilliams JP, Durazo F, El-Kabany M, Kaldas F, et al. Transjugular liver biopsy: safe even in patients with severe coagulopathies and multiple biopsies. Clin Transl Gastroenterol. 2019;10(7):e00063.
doi pubmed - Machado NO. Hemobilia post liver biopsy: Mechanism, presentation, complications and management. Journal of Gastroenterology, Pancreatology & Liver Disorders. 2015;2(4):1-14.
doi - Jahagirdar V, Somashekar U, Verma S, Gunta SP, Banode P, Rama K. S3598 hemobilia with obstructive jaundice in a patient with healing hepatic abscess: a rare presentation. The American Journal of Gastroenterology. 2021;116:S1475.
doi - Albuquerque W, Arantes V, de Paula Farah K, Lambertucci JR. Acute pancreatitis and acute cholecystitis caused by hemobilia after percutaneous ultrasound-guided liver biopsy. Endoscopy. 2005;37(11):1159-1160.
doi pubmed - Redman HC, Joseph RR. Hemobilia and pancreatitis as complication of a percutaneous transhepatic cholangiogram. Am J Dig Dis. 1975;20(7):691-698.
doi pubmed - Chamarthy P, Wong B, Arakali S, Hogshire L. Liver biopsy-induced hemobilia complicated by cholangitis and cholecystitis. American Journal of Gastroenterology. 2015;110(S1):S77.
- Zhornitskiy A, Berry R, Han JY, Tabibian JH. Hemobilia: historical overview, clinical update, and current practices. Liver Int. 2019;39(8):1378-1388.
doi pubmed - Kim KH, Kim TN. Etiology, clinical features, and endoscopic management of hemobilia: a retrospective analysis of 37 cases. Korean J Gastroenterol. 2012;59(4):296-302.
doi pubmed - Gurakuqi GC, Stadlbauer V, Portugaller HR, Hogenauer C, Trauner M, Stauber RE. Fatal hemobilia resulting from an iatrogenic arteriobiliary fistula as a rare complication of transjugular liver biopsy. Eur J Gastroenterol Hepatol. 2008;20(1):83-86.
doi pubmed - Chin MW, Enns R. Hemobilia. Curr Gastroenterol Rep. 2010;12(2):121-129.
doi pubmed - Asselah T, Condat B, Sibert A, Rivet P, Lebray P, Bernuau J, Benhamou JP, et al. Haemobilia causing acute pancreatitis after percutaneous liver biopsy: diagnosis by magnetic resonance cholangiopancreatography. Eur J Gastroenterol Hepatol. 2001;13(7):877-879.
doi pubmed - Srivastava DN, Sharma S, Pal S, Thulkar S, Seith A, Bandhu S, Pande GK, et al. Transcatheter arterial embolization in the management of hemobilia. Abdom Imaging. 2006;31(4):439-448.
doi pubmed - Green MH, Duell RM, Johnson CD, Jamieson NV. Haemobilia. Br J Surg. 2001;88(6):773-786.
doi pubmed - Sandblom P, Mirkovitch V. Minor hemobilia. Clinical significance and pathophysiological background. Ann Surg. 1979;190(2):254-264.
doi pubmed - Song JY, Moon JH, Choi HJ, Kim DC, Choi MH, Lee TH, Cha SW, et al. Massive hemobilia following transpapillary bile duct biopsy treated by using a covered self-expandable metal stent. Endoscopy. 2014;46(Suppl 1 UCTN):E161-162.
doi pubmed - Aslinia F, Hawkins L, Darwin P, Goldberg E. Temporary placement of a fully covered metal stent to tamponade bleeding from endoscopic papillary balloon dilation. Gastrointest Endosc. 2012;76(4):911-913.
doi pubmed - Patel IJ, Rahim S, Davidson JC, Hanks SE, Tam AL, Walker TG, Wilkins LR, et al. Society of interventional radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions-part II: recommendations: endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019;30(8):1168-1184.e1161.
doi pubmed - Marynissen T, Maleux G, Heye S, Vaninbroukx J, Laleman W, Cassiman D, Verslype C, et al. Transcatheter arterial embolization for iatrogenic hemobilia is a safe and effective procedure: case series and review of the literature. Eur J Gastroenterol Hepatol. 2012;24(8):905-909.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, including commercial use, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.