| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com |
Case Report
Volume 16, Number 12, December 2025, pages 499-503
Aortic Dissection During Diagnostic Coronary Angiography in a Patient With Acute Coronary Syndrome: The Role of Off-Pump Coronary Artery Bypass Grafting
Carlos A. Roldana, b, d, Shazib Sagheera, Kathleen Allenb, Lori Serklandb, Breandan Kellya, b, Said Yassinc
aDivision of Cardiology, University of New Mexico School of Medicine, Albuquerque, NM, USA
bDivision of Cardiology, New Mexico VA Health Care System, Albuquerque, NM, USA
cDivision of Cardiothoracic Surgery, New Mexico VA Health Care System, Albuquerque, NM, USA
dCorresponding Author: Carlos A. Roldan, Division of Cardiology, University of New Mexico School of Medicine, Albuquerque, NM 87131, USA
Manuscript submitted September 29, 2025, accepted October 21, 2025, published online November 22, 2025
Short title: Aortic Dissection During Diagnostic Angiography
doi: https://doi.org/10.14740/jmc5214
| Abstract | ▴Top |
Iatrogenic aortic dissection (IAD) is an extremely rare complication of coronary angiography, most often occurring during percutaneous coronary intervention (PCI) and typically resulting from retrograde extension of a coronary artery dissection. The coexistence of IAD with acute coronary syndrome (ACS) presents significant management challenges, with no established guidelines to guide therapy. Management decisions are influenced by several factors, including whether angiography is performed in the setting of stable angina versus ACS, the extent of ischemic myocardium at risk, the patient’s hemodynamic stability, and the extent of aortic involvement - particularly whether the dissection is confined to the aortic root or extends into the ascending aorta by less than or greater than 4 cm. Treatment strategies may include PCI alone for the coronary dissection or urgent surgical repair of the aorta with coronary artery bypass grafting (CABG). The role of off-pump CABG in this context remains poorly defined. We describe a unique case of a patient with severe mid-left anterior descending (LAD) artery disease presenting with a non-ST elevation myocardial infarction (NSTEMI), who developed a type II IAD during diagnostic coronary angiography without associated coronary artery dissection. Prompt recognition, careful imaging with transesophageal echocardiography (TEE) and computed tomography angiography (CTA), and immediate medical stabilization prevented progression of the dissection. This allowed for a successful and uncomplicated off-pump left internal mammary artery (LIMA) CABG to the LAD, resulting in excellent short- and long-term clinical outcomes.
Keywords: Aortic dissection; Coronary angiography; Left anterior descending artery; Acute coronary syndrome; Off-pump coronary artery bypass grafting
| Introduction | ▴Top |
Iatrogenic aortic dissection (IAD) is an exceedingly rare complication of diagnostic coronary angiography, with an overall reported incidence of 0.01% to 0.07% [1-3]. The risk is higher during percutaneous coronary intervention (PCI), with an incidence ranging from 0.05% to 0.12% [1-4]. The primary mechanism involves retrograde extension of a coronary artery dissection into the aorta. If unrecognized and untreated promptly, IAD during coronary angiography or PCI is associated with significant morbidity and mortality [1-3, 5].
While guidelines recommend high-risk, complex surgical interventions for spontaneous type II aortic dissection, there are no specific guidelines for managing IAD during coronary procedures; thus, management strategies are variable and ill-defined [6-10]. The approach to treatment depends on several factors, including whether the angiography was performed for stable angina versus acute coronary syndrome (ACS), the extent of ischemic myocardium at risk, the patient’s hemodynamic stability, and whether the dissection is limited to the aortic root or extends into the ascending aorta by less or greater than 4 cm [1-3]. Treatment options range from coronary stenting alone to urgent or emergent surgical repair, including coronary artery bypass grafting (CABG) of culprit and non-culprit vessels.
Limited data exist on managing patients with non-ST elevation myocardial infarction (NSTEMI) complicated by IAD in the absence of associated coronary artery dissection. Of particular interest is the role of off-pump CABG in individuals with IAD and left anterior descending (LAD) artery-related ACS, an area lacking established guidelines.
| Case Report | ▴Top |
Investigations and diagnosis
A 67-year-old man with a medical history of gastroesophageal reflux disease, hyperlipidemia, and osteoarthritis presented with chest pain, positive troponins, and a normal electrocardiogram. He was diagnosed with NSTEMI and received an oral aspirin loading dose of 324 mg and unfractionated heparin infusion prior to coronary angiography.
After an initial selective angiogram of the non-diseased right coronary artery (RCA), a single successful attempt to engage the left main coronary artery revealed a complex, ≥ 95% stenosis of the mid LAD (Fig. 1a). However, during the last angiogram of the left coronary system, concerns for IAD arose after contrast hang-up or staining was observed on the lateral aortic root wall (Fig. 1b, Supplementary Material 1, jmc.elmerpub.com). Consequently, the plan for PCI of the LAD was aborted, antithrombotic therapy was discontinued, and significant hypertension ranging from 141 - 174/81 - 97 mm Hg, was rapidly normalized to 124 - 126/63 - 72 mm Hg. Patient experienced chest pain at the time of his suspected IAD. This pain resolved with intravenous and intracoronary nitroglycerin and by achieving normotension, suggesting a painful rather than painless IAD [11]. Thereafter, patient remained asymptomatic and hemodynamically stable, prompting an urgent transesophageal echocardiogram (TEE) in the catheterization laboratory.
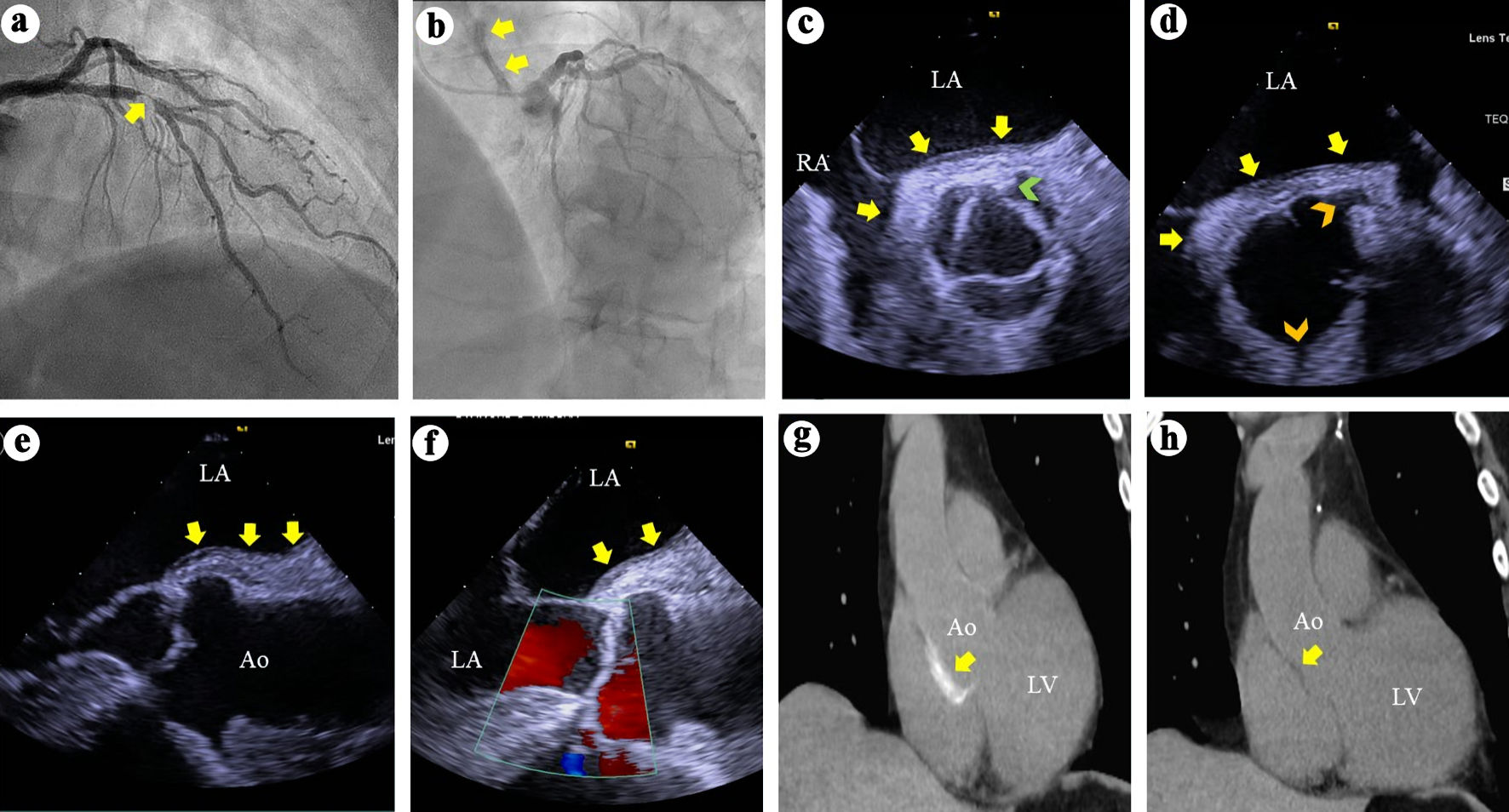 Click for large image | Figure 1. (a) Right anterior oblique view of the left coronary arteries angiography demonstrates a > 95% complex stenosis (arrow) of the left anterior descending coronary artery. (b) Left anterior and cranial view of the left coronary system demonstrates contrast hang-up or staining (arrows) on the aortic root and ascending aorta. (c) TEE short axis views at the aortic valve level during systole demonstrates soft tissue echo-reflectant thickening of the aortic root posterior wall with medial and lateral extensions (arrows) and a possible small intimal tear (green arrowhead). (d) TEE short-axis view of the aortic root during diastole demonstrates aortic wall thickening (arrows) and intact ostial left main and right coronary arteries (top and bottom arrowheads, respectively). (e, f) TEE long-axis views demonstrate soft tissue thickening of the posterior aortic root and ascending aortic wall (arrows) and intact aortic valve with no regurgitation. (g) Thoracic CT demonstrates contrast uptake at the aortic root and part of the ascending aorta (arrow). (h) Repeat thoracic CT 7 weeks later demonstrates resolution of the contrast uptake at the aortic root and ascending aorta. TEE: transesophageal echocardiography; CT: computed tomography; LA: left atrium; RA: right atrium; Ao: aorta; LV: left ventricle. |
TEE showed soft tissue echo-reflectant thickening of the posterolateral aortic root at the level of the non-coronary and left coronary sinuses, with extension of less than 4 cm into the ascending aorta (Fig. 1c-f). A small intimal tear was suspected but not confirmed (Fig. 1c). No dissections involving the left main, LAD, or RCA were identified, and there was no aortic regurgitation (Fig. 1d, e).
Subsequently, a computed tomography angiogram (CTA) confirmed a small intramural hematoma primarily involving the posterior aortic root, extending less than 4 cm into the ascending aorta (Fig. 1g). Immediate surgical consultation was obtained.
Treatment
Through a Heart Team approach, given the patient’s NSTEMI and severe LAD disease, it was decided that he required revascularization, provided his IAD remained stable. PCI was deemed riskier than off-pump CABG using the left internal mammary artery (LIMA) to the LAD. Antiplatelet and anticoagulant therapy were held for 24 h. Aspirin and unfractionated heparin were restarted 24 and 48 h later, respectively, until the day of CABG. The patient’s condition and the stability of his IAD persisted after a week of inpatient observation, and he subsequently underwent an uncomplicated off-pump LIMA to LAD bypass.
Follow-up
Postoperatively, he had an uneventful recovery and was discharged on day 6 in stable condition with guideline-directed medical therapy, including dual antiplatelet therapy (aspirin and clopidogrel). He remained well on outpatient follow-up at weeks 2 and 6. A repeat CTA at week 7 demonstrated resolution of the intramural hematoma (Fig. 1h). He has experienced no recurrent events and remains clinically stable 4 years after presentation.
| Discussion | ▴Top |
This case illustrates a patient with severe mid-LAD-related NSTEMI who developed an IAD during diagnostic coronary angiography without associated coronary dissection. Successful management involved prompt recognition of IAD, cessation of antithrombotic therapy, maintenance of normotension, detailed imaging with TEE and CTA, immediate surgical consultation, close inpatient monitoring, and a collaborative Heart Team decision-making process.
Of particular significance is the use of off-pump LIMA CABG to the LAD as the optimal revascularization strategy in a stabilized IAD with high-risk ACS. This approach offers the advantages of durable arterial grafting while minimizing the risks associated with aortic manipulation, cannulation, or clamping, which could exacerbate or extend the dissection. However, PCI to the LAD may have been a feasible option after 1 week of clinical stability. The Heart Team - including a general cardiologist, interventional cardiologist, and cardiothoracic surgeon - ultimately decided against this strategy. The interventional cardiologist, in particular, expressed concerns regarding possible underlying aortic root inflammation and residual injury. These concerns included the potential risk of re-injury, especially to the ostial left main artery during engagement with a guiding catheter. Thus, the decision was made to proceed with an off-pump LIMA-LAD CABG.
The precise mechanism underlying this patient’s IAD remains uncertain. Technical challenges during engaging the coronary arteries were absent, and no guiding catheters known to increase IAD risk were used. Additionally, PCI was not performed, making procedural causation less likely. A small aortic root intimal tear was suspected but not confirmed, raising the possibility of a spontaneous intramural hematoma.
The pathobiology predisposing to IAD is poorly understood. While cystic medial necrosis has been proposed in some cases, similar histologic findings are also observed in age-matched individuals without dissection. Inflammation within the aortic root and coronary arteries in ACS may predispose to catheter-induced injury, explaining why most IADs (73-83%) occur in ACS patients [12-14].
Upon review of the literature, three retrospective studies highlight the incidence, risk factors, mechanisms, management, and outcomes of IAD (Table 1) [1-3].
 Click to view | Table 1. Iatrogenic Aortic Dissection (IAD): Insights From Three Retrospective Studies |
These three studies provided the following key insights of IAD: 1) Its incidence is rare but increasing slightly in more recent and larger studies (ranging from 0.02% to 0.07%); 2) PCI for ACS is the most common setting; 3) The RCA is most often involved due to its anatomical course and catheter manipulation; 4) Retrograde extension from the coronary artery into the ascending aorta is the typical pathophysiologic mechanism; 5) Management strategies vary with severity: conservative therapy and PCI are often effective and surgical intervention is reserved for extensive dissections (especially > 4 cm), though it may be associated with worse outcomes; and 6) Mortality may be low (about 2.7% in the largest study), suggesting favorable prognosis with prompt recognition and tailored management.
Only two previous cases involving off-pump LIMA CABG for IAD have been documented: Bapat et al [15] reported a woman with LAD dissection extending into the aorta, successfully treated with off-pump LIMA to LAD; and Ahn et al [16] described a similar case and approach in an elderly woman with NSTEMI.
Our case differs from those two reports in that the IAD occurred during diagnostic angiography without PCI and without coronary dissection, highlighting the rarity of our patient’s case.
It is important to note that off-pump LIMA CABG to the LAD in a stabilized patient with IAD and significant LAD disease is not an established recommendation due to limited existing data. However, we believe this approach may be a reasonable option in carefully selected cases, such as ours.
Finally, and of clinical importance, the management of antiplatelet therapy and the optimal waiting time prior to CABG in patients with IAD are not specifically addressed in the current literature and therefore remain undefined. Understandably, the timing and choice of antiplatelet therapy and timing of CABG may vary based on patient’s clinical status, coronary anatomy, and characteristics of the IAD. Patients with ACS and IAD due to retrogradely extended coronary artery dissection treated conservatively with stenting of the dissected coronary artery may require continuation of standard dual antiplatelet therapy. However, patients requiring urgent or emergent CABG - particularly when there is significant myocardium at risk and ongoing hemodynamic instability despite bailout stenting - antiplatelet therapy may need to be withheld. In contrast, clinically stable patients with ACS and IAD without coronary artery dissection may allow for temporary discontinuation of antiplatelet therapy for 24 - 48 h, followed by elective, and preferably off-pump, CABG after 5 - 7 days of conservative therapy.
Conclusions
Although extremely rare, IAD can occur during diagnostic coronary angiography. Prompt recognition, accurate diagnosis, and immediate management are critical to prevent progression and reduce the need for high-risk surgical interventions. In patients with high-risk LAD-related ACS and stable IAD, off-pump LIMA CABG may be a safe and effective revascularization option, minimizing aortic manipulation risks and providing durable coronary repair.
Learning points
IAD is a rare complication that can occur during diagnostic coronary angiography, even in the absence of coronary artery dissection. Early recognition, supported by multimodality imaging, is crucial for accurate diagnosis. Initial conservative medical management may help prevent progression and promote stabilization. In patients with a stabilized IAD and an LAD-related ACS, off-pump CABG using the LIMA artery may provide a safe and effective revascularization strategy.
| Supplementary Material | ▴Top |
Suppl 1. Left anterior and cranial view of the left coronary system suggesting contrast hang-up or staining of the aortic root and ascending aorta.
Acknowledgments
None to declare.
Financial Disclosure
The authors received no financial support for the research, authorship, and/or publication of this manuscript.
Conflict of Interest
The authors declare no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Informed Consent
Our Institutional Review Board does not require informed consent for the report of an individual case report with anonymized data.
Author Contributions
Carlos A. Roldan, MD: Participated in patient’s management and wrote and approved the final manuscript. Shazib Sagheer, MD: Participated in patient’s management and reviewed and edited the manuscript. Kathleen Allen, MD: Performed patient’s coronary angiography, participated in patient’s management, and reviewed and edited the manuscript. Lori Serkland, MD: Performed patient’s transesophageal echocardiogram, participated in patient’s management, and reviewed and edited the manuscript. Breandan Kelly, MD: Reviewed and edited the manuscript. Said Yassin, MD: Performed patient’s CABG and reviewed and edited the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Dunning DW, Kahn JK, Hawkins ET, O'Neill WW. Iatrogenic coronary artery dissections extending into and involving the aortic root. Catheter Cardiovasc Interv. 2000;51(4):387-393.
doi pubmed - Gomez-Moreno S, Sabate M, Jimenez-Quevedo P, Vazquez P, Alfonso F, Angiolillo DJ, Hernandez-Antolin R, et al. Iatrogenic dissection of the ascending aorta following heart catheterisation: incidence, management and outcome. EuroIntervention. 2006;2(2):197-202.
pubmed - Nunez-Gil IJ, Bautista D, Cerrato E, Salinas P, Varbella F, Omede P, Ugo F, et al. Incidence, management, and immediate- and long-term outcomes after iatrogenic aortic dissection during diagnostic or interventional coronary procedures. Circulation. 2015;131(24):2114-2119.
doi pubmed - Huenges K, Dreyer J, Panholzer B, Grothusen C, Renner J, Schafer P, Freundt M, et al. Iatrogenic catheter-induced acute aortic dissection type A after coronary angiography-a retrospective consecutive case series. Thorac Cardiovasc Surg. 2017;65(2):85-89.
doi pubmed - Braverman AC. Acute aortic dissection: clinician update. Circulation. 2010;122(2):184-188.
doi pubmed - Aoyama T, Kunisawa S, Fushimi K, Sawa T, Imanaka Y. Comparison of surgical and conservative treatment outcomes for type a aortic dissection in elderly patients. J Cardiothorac Surg. 2018;13(1):129.
doi pubmed - Koza Y, Kaya U, Tas H, Koza EA. Spontaneous Early Resolution of an Iatrogenic Type A Aortic Dissection Following Coronary Angiography. Aorta (Stamford). 2018;6(6):142-144.
doi pubmed - Doyle B, Juergens CP. Conservative management of ascending aortic dissection caused by percutaneous coronary intervention. J Invasive Cardiol. 2004;16(2):92-94.
pubmed - Eshraghi A, Jalalyazdi M, Ramezani J, Baburian M. Late presentation of iatrogenic dissection of right coronary cusp: a case report. J Cardiovasc Thorac Res. 2020;12(4):341-344.
doi pubmed - Almansori M, Alhulaimi N. Aortic dissection secondary to coronary artery intervention. Neth Heart J. 2010;18(5):264.
doi pubmed - Sonaglioni A, Lombardo M, Rigamonti E, Nicolosi GL, Trevisan R, Zompatori M, Anza C. An unusual case of painless type A aortic dissection. J Clin Ultrasound. 2021;49(7):682-685.
doi pubmed - Wallsh E, Weinstein GS, Franzone A, Clavel A, Rossi PA, Kreps E. Inflammation of the coronary arteries in patients with unstable angina. Tex Heart Inst J. 1986;13(1):105-108.
pubmed - Chandy E, Ivanov A, Dabiesingh DS, Grossman A, Sunkesula P, Velagapudi L, Sales VL, et al. Systemic involvement in ACS: Using CMR imaging to compare the aortic wall in patients with and without acute coronary syndrome. PLoS One. 2018;13(12):e0203514.
doi pubmed - Taglieri N, Nanni C, Ghetti G, Bonfiglioli R, Saia F, Bacchi Reggiani ML, Lima GM, et al. Relation between thoracic aortic inflammation and features of plaque vulnerability in the coronary tree in patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention. An FDG-positron emission tomography and optical coherence tomography study. Eur J Nucl Med Mol Imaging. 2017;44(11):1878-1887.
doi pubmed - Bapat VN, Venn GE. A rare case of aortocoronary dissection following percutaneous transluminal coronary angioplasty: successful treatment using off-pump coronary artery bypass grafting. Eur J Cardiothorac Surg. 2003;24(2):312-314.
doi pubmed - Ahn S, Lee H, Choi JS, Moon Y, Kim IS, Choi SY, Kang JK. Left anterior descending artery dissection with retrograde aortic dissection during percutaneous coronary intervention: a case report. Front Surg. 2023;10:1236734.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.