| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com |
Case Report
Volume 16, Number 9, September 2025, pages 352-359
Ketofol (Ketamine-Propofol) in Pediatric Awake Neurosurgery: An Anesthetic Perspective
Asead Abdylia, Gentian Hutia, b, Mirel Gradaa, Vojsava Lekaa, Stela Dodaja, Florian Dashia, b, Filadelfo Coniglionea, b, c, Krenar Lilajb, Alma Canib, Alert Drishtib, Mustafa Bajraktaria, b, Majlinda Nacob, Alma Soxhukub, Rudin Domia, b, d
aDepartment of Anesthesia and Intensive Care, American Hospital, Tirana, Albania
bDepartment of Surgery, Service of Anesthesia and Intensive Care, University of Medicine, Tirana, Albania
cDepartment of Clinical Sciences and Translational Medicine, Tor Vergata University of Rome, Rome, Italy
dCorresponding Author: Rudin Domi, Department of Surgery, Service of Anesthesia and Intensive Care, University of Medicine, Tirana, Albania
Manuscript submitted July 28, 2025, accepted August 25, 2025, published online September 17, 2025
Short title: Ketofol in Pediatric Awake Neurosurgery
doi: https://doi.org/10.14740/jmc5178
| Abstract | ▴Top |
Awake neurosurgical procedures for brain tumor resections are uncommon in the pediatric population, and careful consideration is required regarding the patient’s cognitive maturity, emotional readiness, and ability to cooperate throughout the intraoperative mapping process. The functional significance of the tumor location may demand precise neurological monitoring, while minimizing sedation to maintain patient responsiveness during cortical stimulation and language testing. We present the case of a 14-year-old patient who was diagnosed with a left temporal lobe tumor. Neuroimaging revealed a lesion with radiological characteristics and clinical correlation highly suggestive of a low-grade glioma. The tumor was situated within the dominant hemisphere, near eloquent cortical regions critically involved in language processing and memory function. These anatomical considerations posed a significant challenge to achieving maximal resection while minimizing the risk of neurological deficits. After thorough multidisciplinary discussion, the neurosurgical team opted for an awake craniotomy. This approach was chosen to facilitate intraoperative cortical and subcortical functional mapping, allowing real-time monitoring of language and cognitive functions. The primary objective was to achieve the greatest possible extent of safe tumor resection while preserving essential neurological functions and ensuring the patient’s long-term quality of life. Anesthetic management of this patient was particularly challenging, as intraoperative seizures were a major concern due to both the tumor’s cortical irritability and the stimulation required for functional mapping. We administered a combination of propofol and ketamine (ketofol) to provide monitored anesthesia care during the procedure. Preoperative planning included seizure prophylaxis, clear communication with the neurosurgical and neuropsychology teams, and the development of contingency strategies for airway management in the event that conversion to general anesthesia became necessary. This case underscores the complexity of pediatric awake craniotomy and highlights the importance of a multidisciplinary, individualized approach to optimize patient safety and surgical outcomes.
Keywords: Pediatric neuroanesthesia; Awake neurosurgery; Ketamine
| Introduction | ▴Top |
Awake craniotomy (AC) is a specialized surgical technique frequently employed to maximize tumor resection in cases where the lesion is in brain regions critical for speech and motor function. By keeping the patient awake during key parts of the procedure, surgeons can perform real-time functional mapping and monitoring, allowing them to identify and preserve essential brain areas responsible for language, movement, and other vital neurological functions. This approach helps maximize the extent of lesion removal while minimizing the risk of postoperative neurological deficits, ultimately improving patient outcomes and quality of life. In selected cases, this technique may also be applied to supratentorial tumors without the need for mapping, aiming to reduce hospital and intensive care unit stays, minimize the risks associated with general anesthesia, lower healthcare costs, and promote the feasibility of ambulatory neurosurgery [1].
Pediatric neuroanaesthesia represents a distinct subspecialty that demands expertise in both pediatric and neuroanesthetic principles. Children undergoing neurosurgical procedures exhibit age-dependent physiological and anatomical variations that significantly influence perioperative management strategies. These differences underscore the need for a specialized approach distinct from adult neuroanaesthesia [2]. The anesthetic care of pediatric neurosurgical patients encompasses unique challenges in pharmacology, airway management, hemodynamic control, intraoperative monitoring, and postoperative neurological assessment. Moreover, communication barriers in younger children often complicate perioperative neurological evaluation. Although the fundamental principles of neuroanesthesia such as maintaining cerebral perfusion, controlling intracranial pressure, ensuring adequate neuromonitoring, and promoting early postoperative recovery are like those in adults, their application in the pediatric population requires a careful adaptation [3]. Further considerations include age-specific variations in airway anatomy, vascular access challenges, differential blood volume management, and tailored anesthetic drug dosing. These factors collectively differentiate pediatric neuroanaesthesia from its adult counterpart and highlight the importance of dedicated training and experience in this field. In our institution, we adapted total intravenous anesthesia using the combination of ketamine and propofol and monitored anesthesia care (MAC) protocol. In addition, ketamine has proven to be both safe and effective in pediatric patients across diverse clinical contexts, reinforcing its utility as a flexible anesthetic agent in neuroanesthesia and critical care practice [4]. We present the case of a 14-year-old patient with a left temporal lobe low-grade glioma who underwent AC for maximal safe resection. Anesthetic management with a ketamine-propofol infusion ensured stable sedation, preserved spontaneous ventilation, and enabled reliable intraoperative mapping. This case highlights the feasibility and benefits of ketamine-propofol combination in the challenging context of pediatric AC.
| Case Report | ▴Top |
Investigations
A 14-year-old male (his weight was 45 kg and height 155 cm), previously healthy and physically active as a soccer player, was referred to our center with radiological findings suggestive of a low-grade glioma in the dominant temporal lobe. He was born at term after an uneventful pregnancy and had normal psychomotor development. Ten days prior to admission, he began experiencing partial epileptic seizures, which prompted imaging studies confirming the diagnosis. Preoperative evaluation, including detailed neurological and psychological assessments, demonstrated no contraindications for AC. Given the tumor’s location within eloquent cortex, the surgical plan consisted of resection under the MAC technique with intraoperative language and motor mapping to maximize safe tumor removal.
Diagnosis
Upon arrival in the operating room, standard monitors were applied, and premedication with 2 mg of midazolam was administered for anxiolysis and seizure prophylaxis. Anesthesia induction included 60 mg of propofol followed by continuous infusion of ketamine (100 mg) and propofol (500 mg) at a rate of 15 - 20 mL/h (Fig. 1). A radial arterial line was inserted for continuous blood pressure monitoring (Fig. 2). A scalp block with lidocaine 2% was performed by the surgical team, and the patient’s head was positioned in a Mayfield head holder.
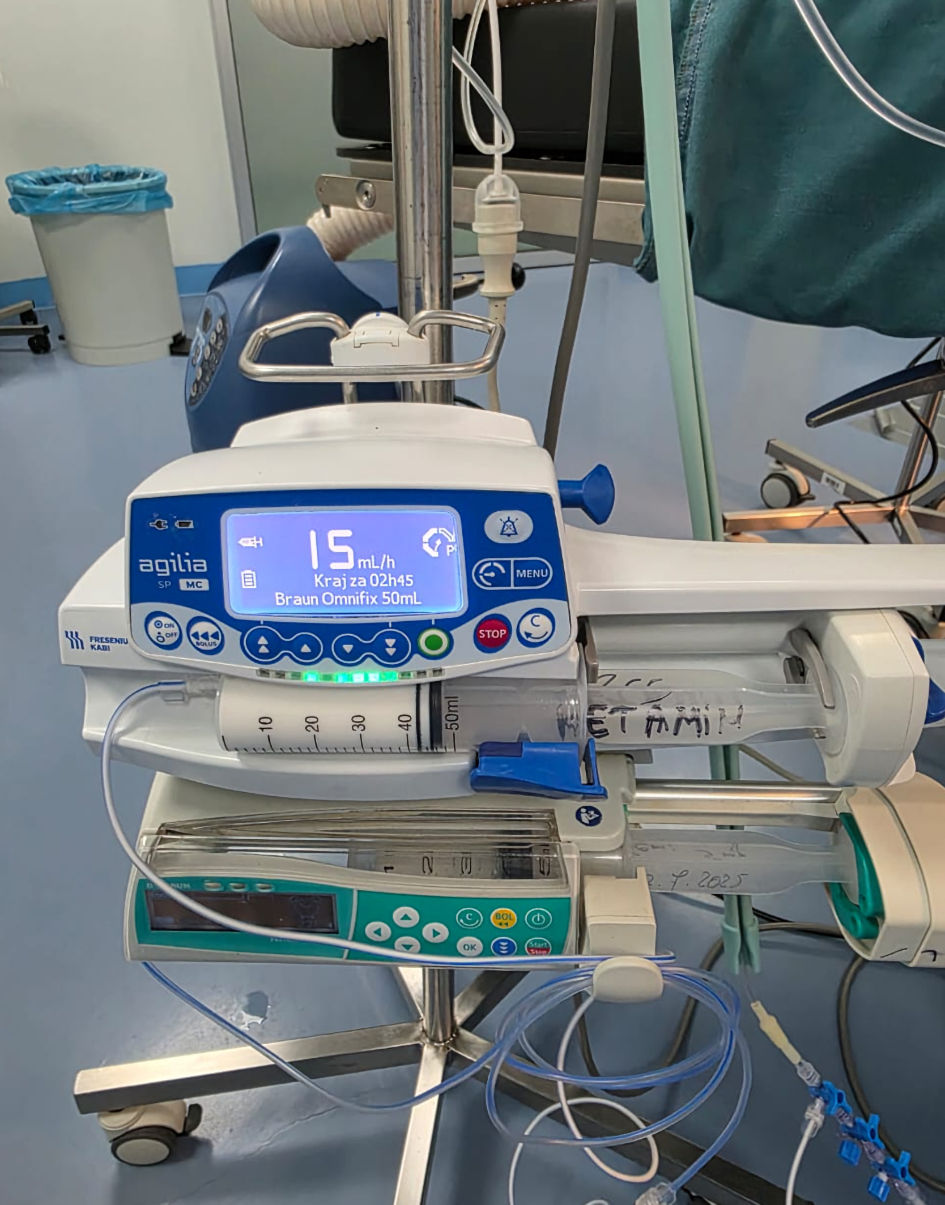 Click for large image | Figure 1. Ketofol (ketamine and propofol) in pump continuous infusion (intraoperative original anesthesia team photo). |
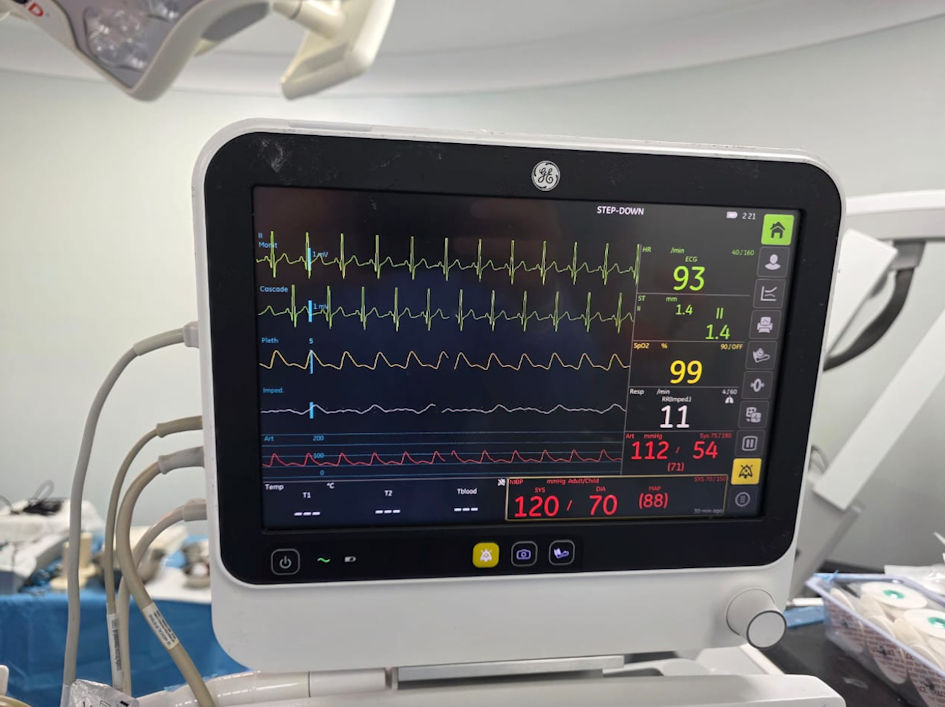 Click for large image | Figure 2. Standard monitoring and invasive blood pressure monitoring through left radial artery cannulation (intraoperative original anesthesia team photo). |
Treatment
The procedure was conducted using the MAC protocol, with supplemental oxygen delivered via a nasal cannula and continuous monitoring to ensure adequate oxygenation and patient safety (Figs. 3-5). After craniotomy and dural opening, anesthetic agents were tapered, and the patient emerged smoothly. Intraoperative neurological examination was conducted by a neurologist, and language mapping was performed through direct cortical stimulation. Tumor resection proceeded without complications or seizure activity. Following tumor removal, anesthesia was reinitiated using the same ketamine-propofol infusion.
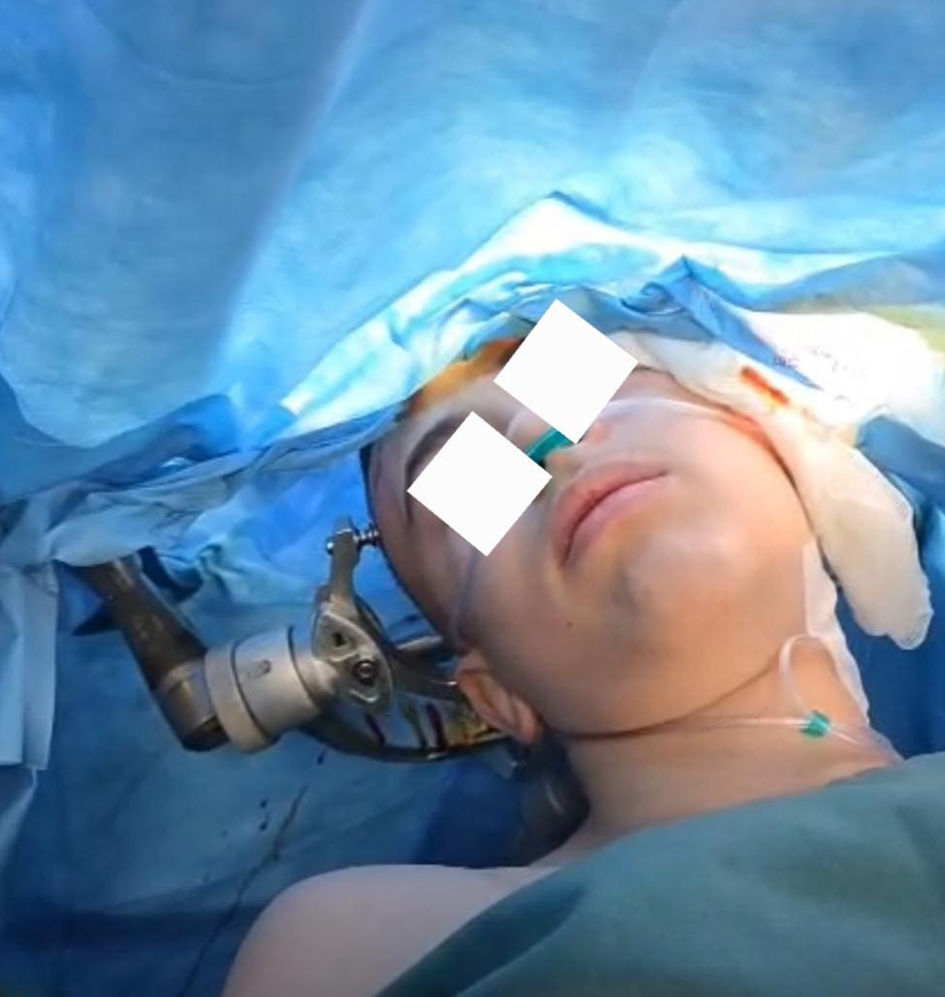 Click for large image | Figure 3. Monitored anesthesia care technique (intraoperative original anesthesia team photo). |
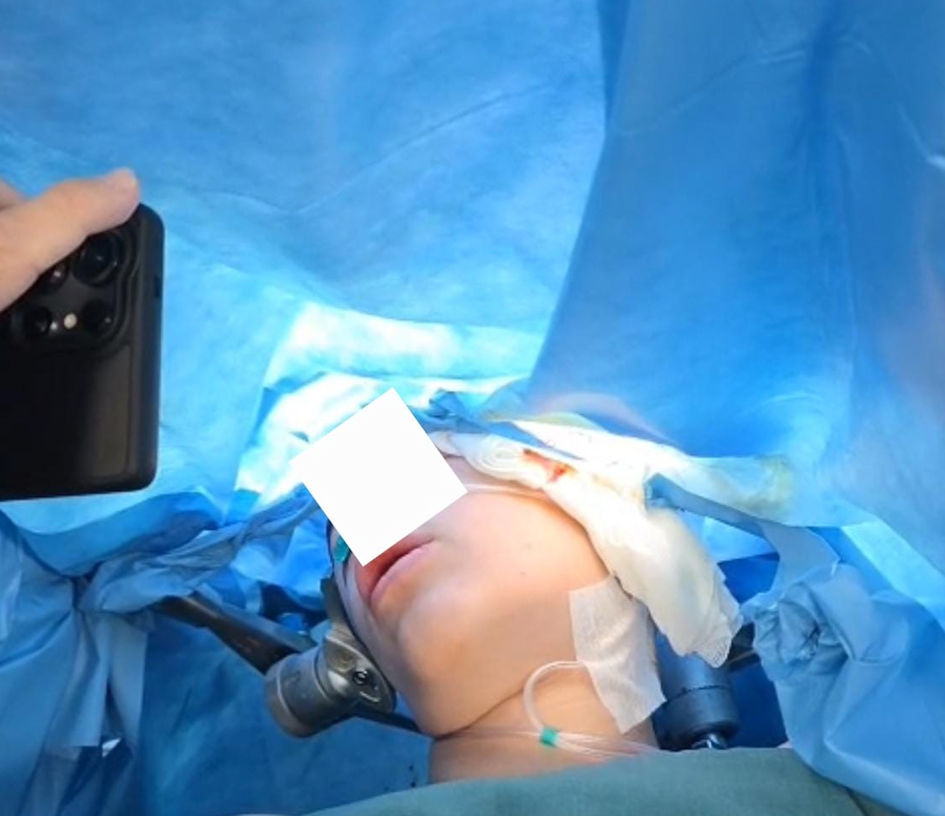 Click for large image | Figure 4. Awake phase and patient interaction with physicians (intraoperative original anesthesia team photo). |
 Click for large image | Figure 5. Patient interaction during awake phase procedures with physicians (intraoperative original anesthesia team photo). |
Follow-up and outcomes
Surgical closure was completed uneventfully. The patient emerged from anesthesia without new neurological deficits and was transferred to the post-anesthesia care unit in stable conditions, before being admitted to the ward for continued monitoring. His postoperative course was favorable, with no complications, and follow-up imaging confirmed adequate tumor resection. He was discharged home in good neurological condition.
| Discussion | ▴Top |
AC represents a highly complex and technically demanding neurosurgical procedure. Its success relies not only on surgical expertise but also on meticulous perioperative management aimed at maximizing efficacy while ensuring patient safety. This necessitates the coordinated involvement of a multidisciplinary team, typically including an anesthesiologist, neurologist, neurophysiologist, and neurosurgeon. Effective and precise communication among team members is critical, particularly during functional mapping and tumor resection, as it directly impacts intraoperative decision-making and neurological outcomes. The multidisciplinary approach allows for real-time assessment of patient function, facilitates the preservation of critical neurological structures, and ultimately enhances the safety and effectiveness of the procedure.
Indications and contraindications of AC
AC is commonly performed to enable intraoperative functional mapping during the resection of brain tumors located near eloquent cortical regions, with the goal of maximizing tumor removal while preserving neurological function in the postoperative period. During AC, the patient remains conscious during the mapping and tumor resection phases, allowing for real-time neurological assessment at critical stages of the procedure. Indications for AC include resection of tumors and epileptogenic foci located in eloquent areas of the cortex regions responsible for motor, sensory, and language functions that are essential to the patient’s daily activities and professional life [5, 6]. Contraindications to AC include sleep apnea, low tolerance to pain, claustrophobic patients, difficult airways, morbid obesity, persistent coughing, uncooperative behavior, and raised intracranial pressure. Table 1 summarizes indications and contraindications of AC.
 Click to view | Table 1. Indications and Contraindications of AC |
Our patient underwent a comprehensive preoperative evaluation, including neurological, anesthetic, and psychological assessments, and was deemed an appropriate candidate for AC. He met all anesthetic and surgical criteria, with imaging and clinical findings confirming the need for resection of a low-grade glioma in the dominant temporal lobe. Considering his young age and active lifestyle as a soccer player, the preservation of cognitive, language, and motor functions was of paramount importance. AC provided the unique advantage of real-time intraoperative functional mapping, allowing the surgical team to identify and preserve eloquent cortical areas while achieving maximal tumor resection. This approach significantly reduces the risk of postoperative neurological deficits, thereby optimizing the patient’s quality of life and increasing the likelihood of returning to high-level physical activity. The procedure was completed uneventfully, and the patient was safely transferred to the ward postoperatively.
| Anesthesia techniques of AC | ▴Top |
There are two main anesthetic approaches for AC: the asleep-awake-asleep (AAA) technique and MAC protocol. Following standard monitoring and local anesthetic infiltration performed by the surgeon, either approach may be selected based on institutional protocols or practitioner preference. The AAA technique involves the induction of general anesthesia with endotracheal intubation or insertion of a laryngeal mask during the initial phase (up to dural opening) and the final phase (after tumor resection and hemostasis). The patient is awakened during the intermediate phase for cortical mapping and tumor resection. In contrast, the MAC technique involves continuous sedation with planned awakening during the second phase for intraoperative mapping and tumor removal, like the AAA approach.
At our center, MAC technique has been utilized in approximately 23 AC procedures between 2019 and 2025, all conducted in accordance with our institutional anesthesia protocol. Notably, this case represents the first application of this approach in the pediatric population at our institution, underscoring both its feasibility and clinical relevance in this age group. Our institutional anesthesia protocol for AC begins with the administration of an intravenous bolus of midazolam (0.03 mg/kg), primarily for anxiolysis and as a prophylactic measure against intraoperative seizures. This is followed by an intravenous bolus dose of propofol (1 mg/kg) to initiate sedation and facilitate patient comfort during the preparatory surgical phase and Mayfield fixing in the skin after local anesthesia made by the surgeon. Subsequently, a continuous infusion of propofol and ketamine is commenced, prepared in a standardized propofol-to-ketamine ratio of 5:1 (corresponding to 500 mg of propofol and 100 mg of ketamine in a single infusion pump). The use of this combination, commonly referred to as “ketofol” provides several clinical advantages. Propofol ensures rapid onset of sedation and favorable recovery characteristics, while ketamine contributes potent analgesia and maintains respiratory drive, thereby reducing the risk of hypoventilation or airway compromise. Moreover, ketamine’s sympathomimetic properties counterbalance the dose-dependent hypotension associated with propofol, promoting greater hemodynamic stability throughout the procedure. The combined effect allows for lighter yet effective sedation, minimizing the need for high doses of either drug alone or ensuring that patients remain cooperative during intraoperative neurological testing. Standard monitoring modalities, including electrocardiography, pulse oximetry, and capnography, were continuously applied, complemented by invasive arterial blood pressure monitoring via radial artery cannulation to enable precise and continuous hemodynamic assessment during all phases of the procedure. After craniotomy and safe dura mater opening, the patient was awakened to enable neurological monitoring and real-time communication with the neurosurgical team. Upon completion of tumor resection and meticulous hemostasis, sedation was resumed for wound closure.
The novel application of ketamine in AC
Ketamine, a phencyclidine-derivative dissociative anesthetic, primarily exerts its pharmacological effects via non-competitive antagonism at N-methyl-D-aspartate (NMDA) receptors, conferring sedation, analgesia, neuroprotection, and antiepileptic activity. Beyond its NMDA receptor blockade, ketamine may also modulate opioid, monoaminergic, and cholinergic pathways, enhancing its multimodal action profile [7]. Historically, ketamine’s use in neurosurgical and neurocritical care has been limited by concerns about increased intracranial pressure (ICP). However, several clinical studies report statistically significant decreases in ICP following ketamine bolus administration in patients with traumatic brain injury (TBI) [8]. These findings have been supported by a systematic review concluding that ketamine does not elevate ICP in sedated, ventilated TBI patients, and may even reduce it in specific cases [8].
A further advantage of ketamine is its stimulatory effect on the sympathetic nervous system, which supports systemic blood pressure and cardiac output, thereby preserving cerebral perfusion pressure particularly critical in hemodynamically compromised patients. Preclinical and clinical research has also highlighted ketamine’s ability to attenuate cortical spreading depolarizations (SDs) pathologic electrophysiological waves associated with secondary neuronal injury. Early electrocorticography in two acute brain injury cases (traumatic and spontaneous intracranial hemorrhage) demonstrated that ketamine inhibited SDs, restoring cortical electrical activity [9, 10]. Table 2 summarizes the advantages of ketamine in AC procedures.
 Click to view | Table 2. Advantages of Ketamine in AC Procedures |
Clinical evaluations of ketofol have demonstrated favorable outcomes. In procedural sedation, ketofol has been shown to maintain better cerebral oxygenation and hemodynamic stability compared to propofol alone [11]. A focused study on AC reported safe and efficient sedation with a 1:1 ketamine-propofol infusion: patients experienced rapid recovery, minimal hemodynamic/respiratory events, high satisfaction, and short post-anesthesia care unit transfer times [12].
AC in adults and in pediatric age
AC has become a cornerstone of modern neurosurgical practice, particularly in the context of functional preservation. Initially developed to enable maximal resection of tumors located within or adjacent to eloquent cortical regions, AC relies on intraoperative electrical stimulation and real-time neurological testing while the patient remains responsive. This strategy markedly decreases the likelihood of permanent neurological deficits while optimizing the extent of tumor removal, an important prognostic factor in diffuse gliomas and other infiltrative brain neoplasms [13].
In recent years, the scope of AC has broadened beyond functional mapping. Reports indicate that in carefully selected cases involving tumors in non-eloquent regions, the procedure may be performed without mapping. The absence of general anesthesia in such cases reduces physiological stress, accelerates recovery, and permits immediate neurological assessment. These advantages closely align with the principles of enhanced recovery after surgery (ERAS) and have made AC an integral component of the evolving paradigm of ambulatory neurosurgery, where discharge within 24 h is feasible under strict perioperative criteria [14]. Patient eligibility is crucial for success. AC is generally indicated for supratentorial lesions involving motor, sensory, or language areas, as well as for epilepsy surgery. Conversely, conditions such as morbid obesity, obstructive sleep apnea, psychiatric instability, poor cooperation, or markedly raised intracranial pressure represent contraindications [5]. Careful preoperative assessment by a multidisciplinary team is therefore mandatory.
Lu et al systematically reviewed the outcomes of AC in pediatric patients [15]. Four observational studies including 57 children (median age 14 years; 60% male) were analyzed. Lesions were predominantly left-sided (80%). Pooled results showed intraoperative complications in 17% of cases, conversion to general anesthesia in 2%, immediate postoperative complications in 18%, and long-term complications in 6%, with seizures being the most common intraoperative issue. Overall, AC in children appears feasible, with complication rates comparable to adults, though certainty is low due to limited data. Careful neuropsychological preparation and follow-up may improve patient selection and outcomes [15].
Fudhaili et la in their systematic review had analyzed 16 studies on pediatric AC, primarily for tumor resection, in children with a mean age of 12.2 years (53% male). Nearly half of the patients achieved complete recovery postoperatively, while 7.5% experienced speech deficits among those with complications. The findings suggest that AC in children is feasible and safe, effectively helping to preserve motor and language functions [16].
Long et al described a 16-year-old male who underwent an AC with language mapping for excision of a left antero-temporal brain arteriovenous malformation. Using an AAA approach and tailored perioperative modifications, the procedure prioritized speech preservation while maintaining patient comfort. The case highlights that AC in children is feasible for select patients, emphasizing multidisciplinary preparation, flexible workflows, and individualized care [17].
Labuschagne et al reported data from an 11-year-old girl with a right motor cortex tumor who underwent a simulated theatre experience to assess her suitability for AC, despite concerns about distractibility. The simulation successfully evaluated her ability to cope, and she subsequently underwent an AAA procedure with cortical and subcortical mapping. She remained cooperative throughout, and postoperative imaging confirmed complete tumor removal. The case demonstrates that preoperative simulation can help identify pediatric candidates for awake surgery, even when initial concerns exist [18].
Interesting data come from the study by O’Leary et al. His group examined eight studies of AC in 85 patients under 18 years of age, focusing on psychological impact. Eligibility was assessed based on cognitive maturity rather than age, and interventions to reduce anxiety varied widely, with no standardized protocols. Most children tolerated the procedure well, though some experienced postoperative disruptions, such as effects on school attendance. The review highlights the need for standardized pre- and post-surgical psychological assessments to monitor anxiety, trauma, and functional recovery in pediatric AC patients [19].
Approximately the same conclusions came from the study by Lohkamp et al, which included 18 pediatric patients (median age 14.8 years), who underwent AAA brain surgery for central nervous system (CNS) lesions. Intraoperative electrocortical mapping enabled functional preservation, with complete tumor removal achieved in 65% of cases. Postoperative neurological deficits were mostly transient, and severe psychological reactions were rare. The findings suggest awake brain surgery is feasible and beneficial in children, highlighting the importance of thorough psychological preparation and preoperative and postoperative neuropsychological testing to monitor cognitive outcomes [20].
Recently Henry et al published their review article regarding AC [21]. They reported that AC with functional cortical mapping is increasingly used in selected pediatric patients to minimize postoperative deficits. Their paper shows “asleep-awake-asleep” anesthesia with agents like propofol and remifentanil is most common. The review concludes that, with careful selection and planning, AC is safe and feasible in children [21].
A retrospective study of 28 children (30 procedures; median age 14) demonstrates that AC with intraoperative mapping is feasible in the pediatric population, with 96.7% of cases completed without conversion to general anesthesia. Serious but transient complications occurred in a small number of patients, and new neurological deficits were mild or moderate, resolving by 6 months. The study highlights the importance of careful patient selection and multidisciplinary collaboration for safe and effective pediatric AC [22].
Multimodal analgesia in AC
Effective scalp anesthesia is a critical component of AC, necessary for multiple phases of the procedure. It ensures patient comfort during skull pinning and skin incision in cases performed under conscious sedation and helps reduce pain during emergence in AAA protocols. Local infiltration with 1-2% lidocaine is commonly used to anesthetize the pin sites prior to head fixation in a Mayfield clamp. However, for more comprehensive and long-lasting analgesia, a regional approach is often preferred [23]. Scalp anesthesia may be achieved either through field infiltration around the surgical incision and pin sites or via a scalp nerve block. The scalp block involves targeted infiltration of local anesthetic around six paired sensory nerves: the supraorbital, supratrochlear, auriculotemporal, zygomaticotemporal, greater occipital, and lesser occipital nerves. This technique provides dense, symmetric analgesia to the entire scalp, significantly improving intraoperative comfort and reducing the need for systemic analgesics or sedatives. Additionally, an effective scalp block can attenuate the hemodynamic response to nociceptive stimuli such as skull pinning and skin incision, contributing to intraoperative cardiovascular stability. Given its safety, simplicity, and efficacy, the scalp block is widely used in both adult and pediatric populations undergoing awake neurosurgical procedures and is often incorporated as a standard component of anesthetic management in institutions performing AC [24, 25]. In our case, the neurosurgeon performed a scalp infiltrative block using diluted 2% lidocaine, targeting the incision site and pinning areas prior to Mayfield head clamp application. This local anesthetic technique provided effective analgesia, enabling atraumatic positioning and reducing patient discomfort during skull pinning. As a result, the need for systemic sedatives and analgesics was minimized during the initial phase of the procedure, contributing to greater hemodynamic stability and facilitating smoother intraoperative neurological monitoring. The use of local infiltration also aligned with our institutional protocol to enhance patient cooperation and minimize the depth of sedation during AC.
ERAS protocols have been recently introduced into neurosurgical practice, with implementation efforts increasingly supported by both anesthesiologists and neurosurgeons. Initially developed and validated in colorectal and other general surgical procedures [26], ERAS protocols are structured, multidisciplinary perioperative care pathways designed to standardize best practices, minimize physiological stress, and promote faster recovery. In the context of neurosurgery, the adaptation of ERAS principles seeks to reduce perioperative complications, enhance patient safety, and shorten hospital length of stay [27, 28]. Key components include preoperative optimization, minimally invasive surgical techniques, goal-directed fluid therapy, multimodal analgesia, and early mobilization [29]. When appropriately applied, these strategies can support the transition of selected neurosurgical procedures such as craniotomies for tumor resection or spinal surgeries toward ambulatory or short-stay models of care [30]. Ongoing research and refinement are essential to tailor ERAS protocols to the unique physiological and neurological considerations inherent to neurosurgical patients [27, 31]. In our patient, a combined approach utilizing scalp infiltration with local anesthetics and an analgesia-sedation regimen was implemented to enhance intraoperative stability, minimize the need for deeper general anesthesia, and improve overall patient comfort. This strategy contributed to optimal surgical conditions, reduced intraoperative stress responses, and supported a smoother and more rapid postoperative recovery.
Limitations
This study has several limitations, including the paucity of available evidence, small patient cohorts, and the predominance of single-center studies. Apart from our own series of adult awake craniotomies, this represents our first reported pediatric case. Accordingly, further studies and additional reports from other centers are warranted to expand the current knowledge base.
Conclusions
AC in pediatric patients is complex, requiring multidisciplinary coordination and careful patient selection. In this case, the use of a combined ketamine-propofol infusion provided adequate sedation while preserving airway reflexes and allowing smooth emergence for neurological assessment. MAC protocol proved effective, facilitating intraoperative mapping without complications. This case demonstrates that AC is feasible and safe in selected pediatric patients with lesions in eloquent brain regions. Careful anesthetic planning and interdisciplinary collaboration are essential for optimizing outcomes in this setting.
Learning points
MAC with ketamine-propofol is a safe and effective strategy in pediatric AC, providing opioid-sparing effects, stable hemodynamics, and favorable conditions for intraoperative mapping and tumor resection.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained.
Author Contributions
RD, AA, KL, MB, MN: write the paper; GH, VL, FC, AC, AS: literature searching; SD, MG, AD, FD: language editing
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Paldor I, Drummond KJ, Awad M, Sufaro YZ, Kaye AH. Is a wake-up call in order? Review of the evidence for awake craniotomy. J Clin Neurosci. 2016;23:1-7.
doi pubmed - Domi R. Emerging trends in paediatric neurosurgical anaesthesia: Time for subspeciality? Indian J Anaesth. 2024;68(9):750-751.
doi pubmed - Domi R, Coniglione F, Abdyli A, Huti G, Lilaj K, Bilotta F. Anaesthesia considerations on paediatric neurosurgery. Turk J Anaesthesiol Reanim. 2025;53(2):34-41.
doi pubmed - Dasari L, Ninave S. A narrative review of the efficacy and safety of oral ketamine in pediatric sedation: a critical analysis of current evidence. Cureus. 2024;16(8):e67550.
doi pubmed - Kim SH, Choi SH. Anesthetic considerations for awake craniotomy. Anesth Pain Med (Seoul). 2020;15(3):269-274.
doi pubmed - Meng L, McDonagh DL, Berger MS, Gelb AW. Anesthesia for awake craniotomy: a how-to guide for the occasional practitioner. Can J Anaesth. 2017;64(5):517-529.
doi pubmed - Domi R, Cani A, Abdyli A, Huti G, Dodaj S, Coniglione F, Grada M, et al. Ketamine in neurocritical care: new potentials and perspectives. Cureus. 2025;17(6):e85456.
doi pubmed - Sameer M, Abbas DA. Clinical outcomes of ketamine in patients with traumatic brain injury: A systematic review. Int J Crit Illn Inj Sci. 2024;14(3):160-175.
doi pubmed - Sakowitz OW, Kiening KL, Krajewski KL, Sarrafzadeh AS, Fabricius M, Strong AJ, Unterberg AW, et al. Preliminary evidence that ketamine inhibits spreading depolarizations in acute human brain injury. Stroke. 2009;40(8):e519-522.
doi pubmed - Telles JPM, Welling LC, Coelho A, Rabelo NN, Teixeira MJ, Figueiredo EG. Cortical spreading depolarization and ketamine: a short systematic review. Neurophysiol Clin. 2021;51(2):145-151.
doi pubmed - Bhaire VS, Panda N, Luthra A, Chauhan R, Rajappa D, Bhagat H. Effect of combination of ketamine and propofol (Ketofol) on cerebral oxygenation in neurosurgical patients: a randomized double-blinded controlled trial. Anesth Essays Res. 2019;13(4):643-648.
doi pubmed - Samar A, Hany A, Enas S, Ehab E. Preliminary evaluation of ketofol-based sedation for awake craniotomy procedures. Egyptian Journal of Anaesthesia. 2010;26:293-297.
doi - Staartjes VE, Hijazi S, Klukowska AM, et al. Safety and cost-effectiveness of awake craniotomy for supratentorial gliomas in a developing country setting. Clin Neurol Neurosurg. 2020;192:105719.
doi - Gerritsen JKW, Vietor CL, Rizopoulos D, Schouten JW, Klimek M, Dirven CMF, Vincent AJE. Awake craniotomy versus craniotomy under general anesthesia without surgery adjuncts for supratentorial glioblastoma in eloquent areas: a retrospective matched case-control study. Acta Neurochir (Wien). 2019;161(2):307-315.
doi pubmed - Lu VM, Maddy K, Niazi TN. Awake craniotomy in pediatric patients: a meta-analysis of operative outcomes. World Neurosurg. 2024;181:154-160.e152.
doi pubmed - Al Fudhaili AN, Al-Busaidi F, Madan ZM, Al Issa MS, Al Mamria MH, Al-Saadi T. Awake craniotomy surgery in pediatrics: a systematic review. World Neurosurg. 2023;179:82-87.
doi pubmed - Long M, Thiaghu C, Cheong TM, Kirollos RW, Han J, Ng LP, Low SYY. Awake craniotomy for the excision of a pediatric cerebral arteriovenous malformation for language preservation: a case description. J Pers Med. 2025;15(7):319.
doi pubmed - Labuschagne J, Lee CA, Mutyaba D, Mbanje T, Sibanda C. Awake craniotomy in a child: assessment of eligibility with a simulated theatre experience. Case Rep Anesthesiol. 2020;2020:6902075.
doi pubmed - O'Leary KD, Philippopoulos AJ, Koslofsky A, Ahmed Y. How often do awake craniotomies in children and adolescents lead to panic and worry? Childs Nerv Syst. 2024;40(2):359-370.
doi pubmed - Lohkamp LN, Beuriat PA, Desmurget M, Cristofori I, Szathmari A, Huguet L, Di Rocco F, et al. Awake brain surgery in children-a single-center experience. Childs Nerv Syst. 2020;36(5):967-974.
doi pubmed - Henry MN, Shahin MN, Stevens I, Calvert J, Colgan DD, Vega M, Collins K. Pediatric awake craniotomy: an educational review. Paediatr Anaesth. 2025;35(4):270-276.
doi pubmed - Alcaraz Garcia-Tejedor G, Echaniz G, Strantzas S, Jalloh I, Rutka J, Drake J, Der T. Feasibility of awake craniotomy in the pediatric population. Paediatr Anaesth. 2020;30(4):480-489.
doi pubmed - Bhanja D, Sciscent BY, Daggubati LC, Ryan CA, Pahapill NK, Hazard SW, Rizk EB. Awake craniotomies in the pediatric population: a systematic review. J Neurosurg Pediatr. 2023;32(4):428-436.
doi pubmed - Potters JW, Klimek M. Local anesthetics for brain tumor resection: current perspectives. Local Reg Anesth. 2018;11:1-8.
doi pubmed - Kulikov A, Tere V, Sergi PG, Bilotta F. Prevention and treatment of postoperative pain in pediatric patients undergone craniotomy: Systematic review of clinical evidence. Clin Neurol Neurosurg. 2021;205:106627.
doi pubmed - Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152(3):292-298.
doi pubmed - Stumpo V, Staartjes VE, Quddusi A, Corniola MV, Tessitore E, Schroder ML, Anderer EG, et al. Enhanced Recovery After Surgery strategies for elective craniotomy: a systematic review. J Neurosurg. 2021;135(6):1857-1881.
doi pubmed - Khozenko A, Lamperti M, Velly L, Simeone P, Tufegdzic B. Role of anaesthesia in neurosurgical enhanced recovery programmes. Best Pract Res Clin Anaesthesiol. 2021;35(2):241-253.
doi pubmed - Aurilio C, Pace MC, Sansone P, Giaccari LG, Coppolino F, Pota V, Barbarisi M. Multimodal analgesia in neurosurgery: a narrative review. Postgrad Med. 2022;134(3):267-276.
doi pubmed - Pelaez-Sanchez CA, Pajaron-Guerrero M, Rodriguez-Caballero A, Ruiz Calderon C, Mora C, Martin-Laez R, Sampedro I, et al. Enhanced recovery and same-day discharge after brain tumor surgery under general anesthesia: initial experience with Hospital-at-Home-based postoperative follow-up. Neurosurg Focus. 2023;55(6):E6.
doi pubmed - Gangakhedkar GR, Monteiro JN, Bedekar U, Desai M. A national online questionnaire-based survey, regarding the awareness and implementation of enhanced recovery after surgery practices in neurosurgical procedures. Indian J Anaesth. 2023;67(Suppl 1):S60-S63.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.