| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com |
Case Report
Volume 16, Number 9, September 2025, pages 337-344
Anesthesiologist’s Concerns About Dandy-Walker Syndrome: Airway Management, Muscle Relaxants, and Train-of-Four Monitoring of Neuromuscular Blockade
Asead Abdylia, Oliatina Demiria, Gentian Hutia, b, Filadelfo Coniglionea, c, Alert Drishtib, Krenar Lilajb, Rudin Domib, c, d
aDepartment of Anesthesiology and Intensive Care, American Hospital, Tirana, Albania
bDepartment of Surgery, University of Medicine, Tirana, Albania
cDepartment of Clinical Science and Translational Medicine, Tor Vergata University of Rome, Rome, Italy
dCorresponding Author: Rudin Domi, Department of Surgery, University of Medicine, Tirana, Albania
Manuscript submitted July 15, 2025, accepted August 19, 2025, published online September 17, 2025
Short title: Anesthesiologist and Dandy-Walker Syndrome
doi: https://doi.org/10.14740/jmc5170
| Abstract | ▴Top |
Dandy-Walker syndrome is a rare congenital malformation of the posterior fossa that presents unique anesthetic challenges, including difficult airway management, altered consciousness, hydrocephalus, and potential for prolonged postoperative ventilation. Hydrocephalus, the most common finding, can complicate ventilation and intubation, whereas brainstem involvement, along with agenesis of the corpus callosum, pontine hypoplasia, and distortion of the medullary respiratory centers, may contribute to prolonged postoperative ventilation. Anesthetic management in such cases requires thorough airway assessment, preparation for potential airway difficulties, intracranial pressure monitoring and control, and total intravenous anesthesia (TIVA) to facilitate rapid emergence. We describe the case of a 3-year-old male with macrocephaly, movement disorders, delayed cognitive development, and altered mental status, scheduled for ventriculoperitoneal shunt placement under TIVA without muscle relaxants, with careful airway preparation and intracranial pressure control. To our knowledge, this is the first reported case of Dandy-Walker syndrome managed in this manner.
Keywords: Dandy-Walker syndrome; Pediatric neuroanesthesia; Intracranial pressure; Airway; Postoperative respiratory complications; Total intravenous anesthesia; Muscle relaxants; Train of four monitoring
| Introduction | ▴Top |
Dandy-Walker syndrome is a rare congenital malformation of the posterior fossa, characterized by hydrocephalus, dilatation of the fourth ventricle, and hypoplasia of the cerebellar vermis. It is associated with increased intracranial pressure and neurological impairment. The estimated incidence is approximately 1 in 30,000 live births [1]. In addition to the characteristic central nervous system abnormalities, patients with Dandy-Walker syndrome may present with a range of associated congenital malformations, most commonly involving the cardiovascular and craniofacial systems. Several studies have documented the coexistence of non-cyanotic congenital heart diseases, such as atrial and ventricular septal defects, as well as more complex lesions, including tetralogy of Fallot. Craniofacial anomalies, along with musculoskeletal abnormalities such as scoliosis, have also been reported, reflecting the multisystem nature of the syndrome and underscoring the need for comprehensive multidisciplinary assessment [2-4].
Hydrocephalus represents the most common neurological manifestation, occurring in approximately 70-90% of cases. This frequently necessitates early neurosurgical intervention due to progressive intracranial hypertension and its adverse effects on neurodevelopment. Encephalocele is reported in about 16% of patients, while structural abnormalities of the corpus callosum, including hypoplasia or complete agenesis, are found in nearly 30%, further contributing to the complexity of the neurodevelopmental profile [4].
Clinically, Dandy-Walker syndrome is characterized by signs of increased intracranial pressure, macrocephaly, headache, nausea, and vomiting, alongside cerebellar signs such as movement disorders, respiratory difficulties, diminished gag reflex, poor nutritional status, and delayed neurodevelopment. Hydrocephalus remains the main surgical indication, with ventriculoperitoneal shunt placement being the standard approach.
We present the case of an infant diagnosed with Dandy-Walker syndrome within the first months of life after seizure onset. The diagnosis was confirmed through imaging and clinical evaluation. The patient underwent ventriculoperitoneal shunt placement under general anesthesia. In this case, particular emphasis was placed on the use of total intravenous anesthesia (TIVA) and the deliberate avoidance of muscle relaxants to address the potential for difficult endotracheal intubation, facilitate rapid emergence, and reduce the risk of postoperative pulmonary complications. This approach underscores the importance of tailoring anesthetic management to the unique neuroanatomical and respiratory challenges presented by Dandy-Walker syndrome.
| Case Report | ▴Top |
Investigations
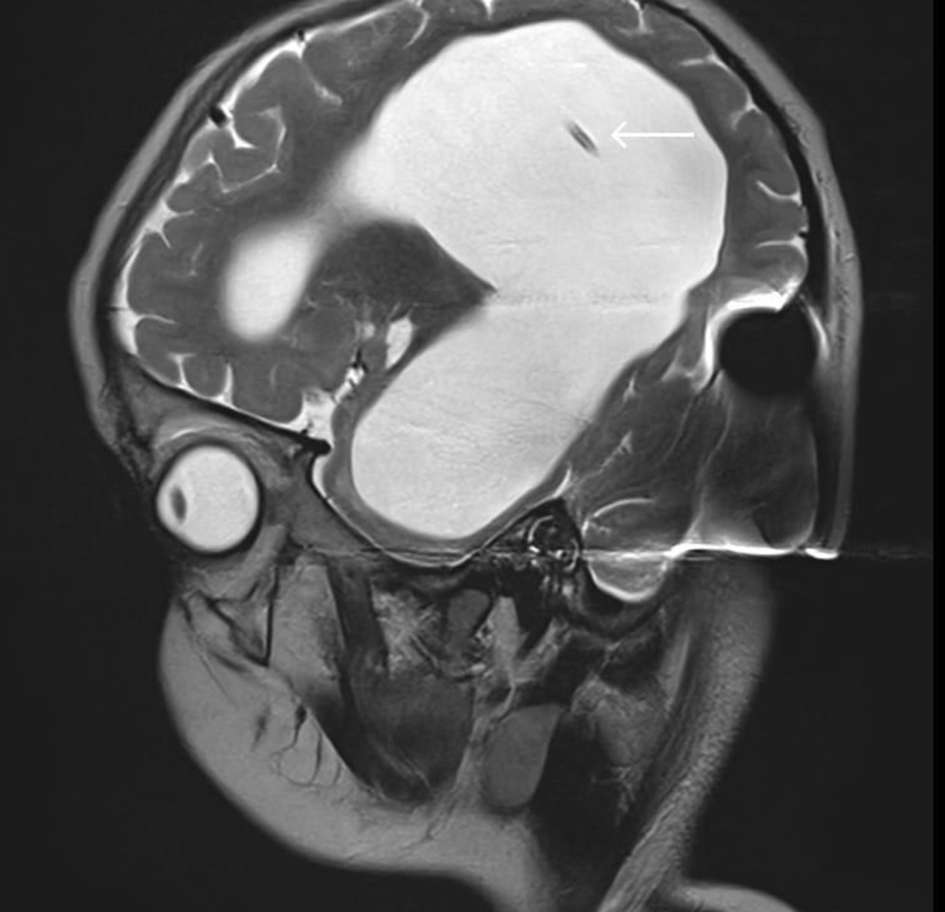
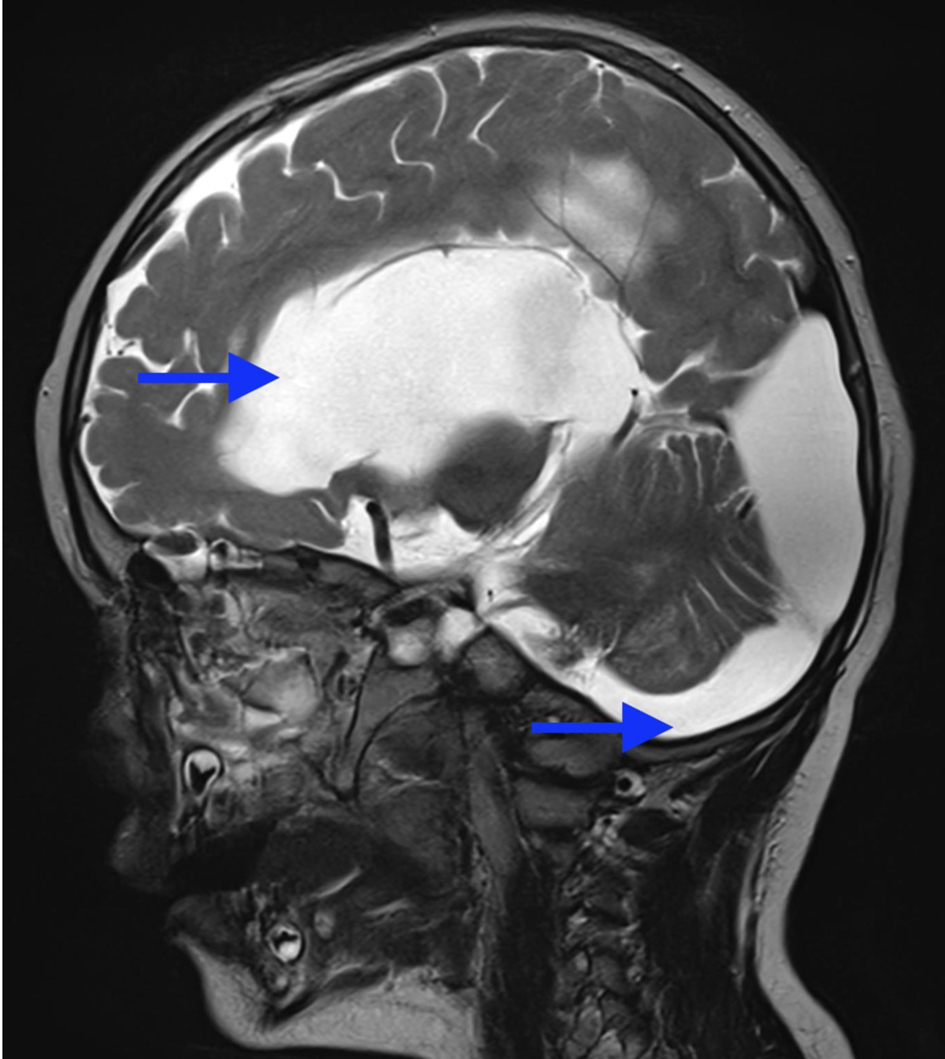
A 3-year-old male patient, weighing 21 kg, was admitted to our institution for surgical management of Dandy-Walker syndrome. The planned procedure was a ventriculoperitoneal shunt placement. The patient was born at term, with the first clinical signs of the condition appearing at 2.5 years of age, prompting referral to a pediatric neurologist and subsequent imaging studies. The diagnosis of Dandy-Walker syndrome was based on the presence of hydrocephalus, dilation of the fourth ventricle, and cerebellar hypoplasia (Figs. 1-3). Magnetic resonance imaging (MRI) concluded on agenesia of vermis, dilatation of the fourth ventricles, and hydrocephaly due to aquaeductus Silvi stenosis.
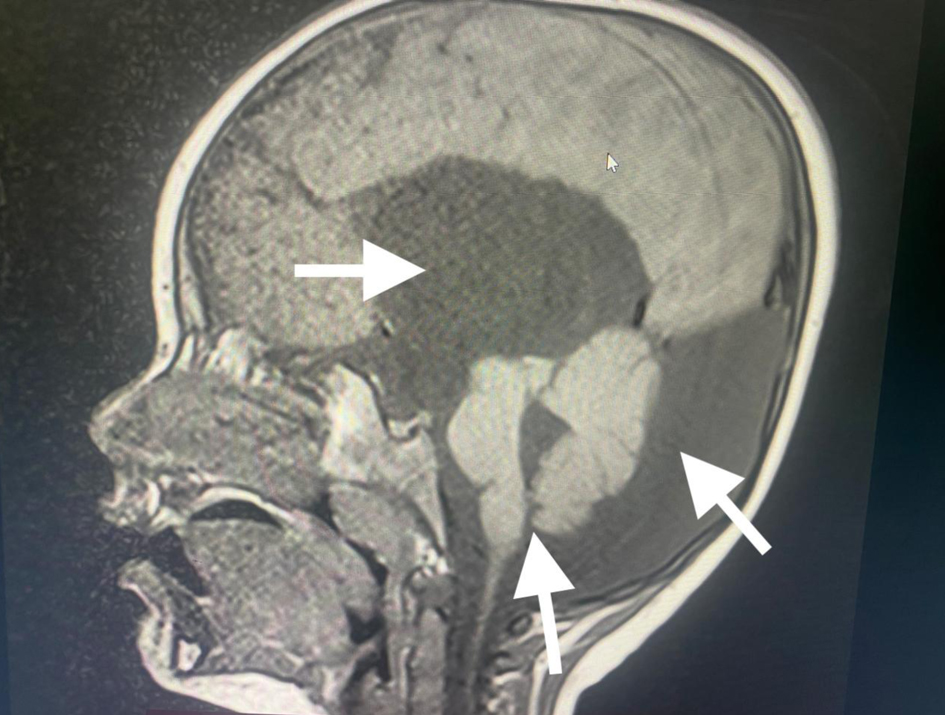 Click for large image | Figure 1. Preoperative head MRI demonstrating agenesis of the cerebellar vermis, dilatation of the fourth ventricle, and hydrocephalus (arrows). MRI: magnetic resonance imaging. |
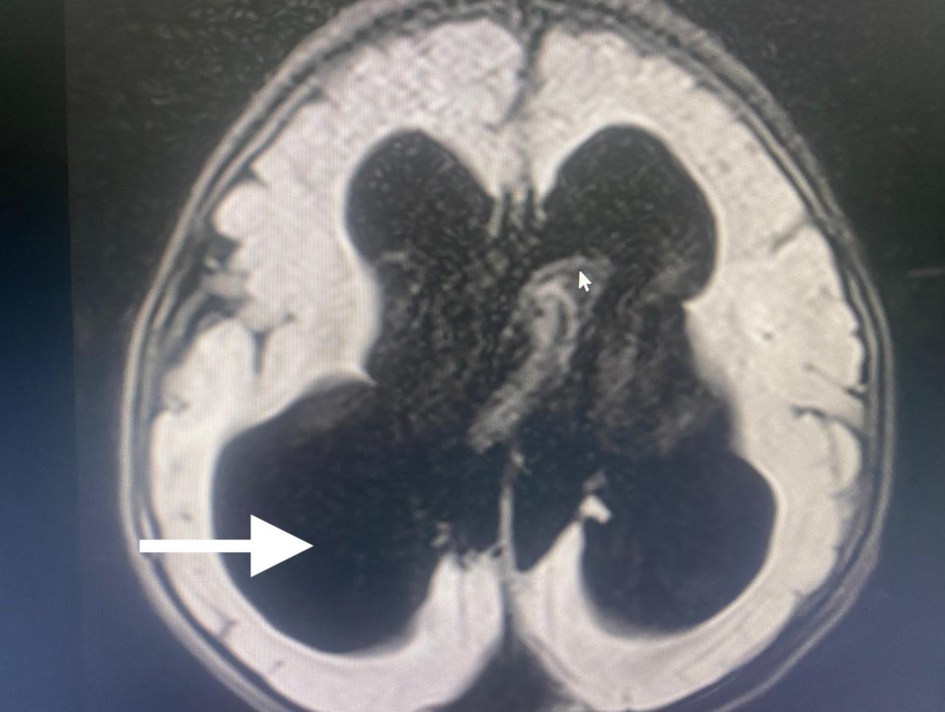 Click for large image | Figure 2. Preoperative head MRI demonstrating significant hydrocephaly (arrow). MRI: magnetic resonance imaging. |
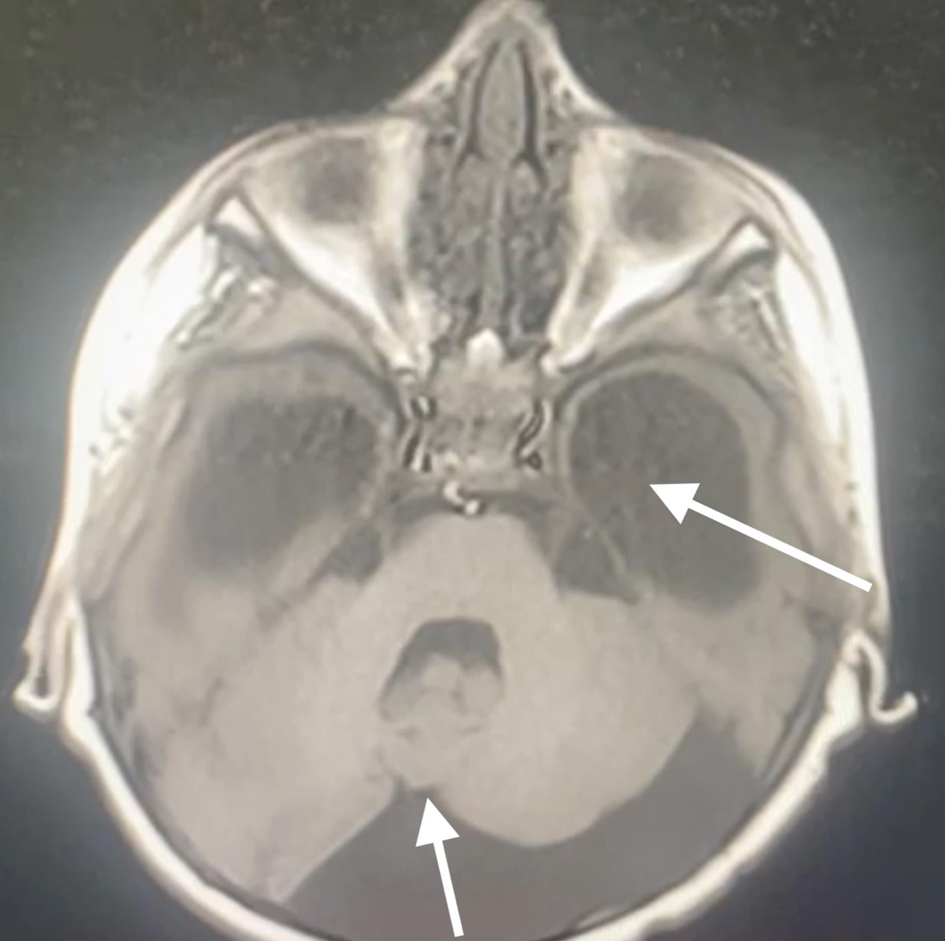 Click for large image | Figure 3. Preoperative head MRI demonstrating agenesis of the cerebellar vermis, significant hydrocephaly (arrows). MRI: magnetic resonance imaging. |
The clinical presentation was dominated by signs of increased intracranial pressure, including agitation, altered mental status, nausea, vomiting, deviated eyes, and impaired motor coordination (Fig. 4). The patient could not follow simple commands, moderate ataxia, and manifested spastic tetra paresis especially in inferior limbs. Pediatric and cardiologic evaluations were unremarkable, and biochemical analyses were within normal limits. Airway assessment was limited by the patient’s lack of cooperation; Mallampati class II-III and mild micrognathia was estimated (Figs. 5 and 6).
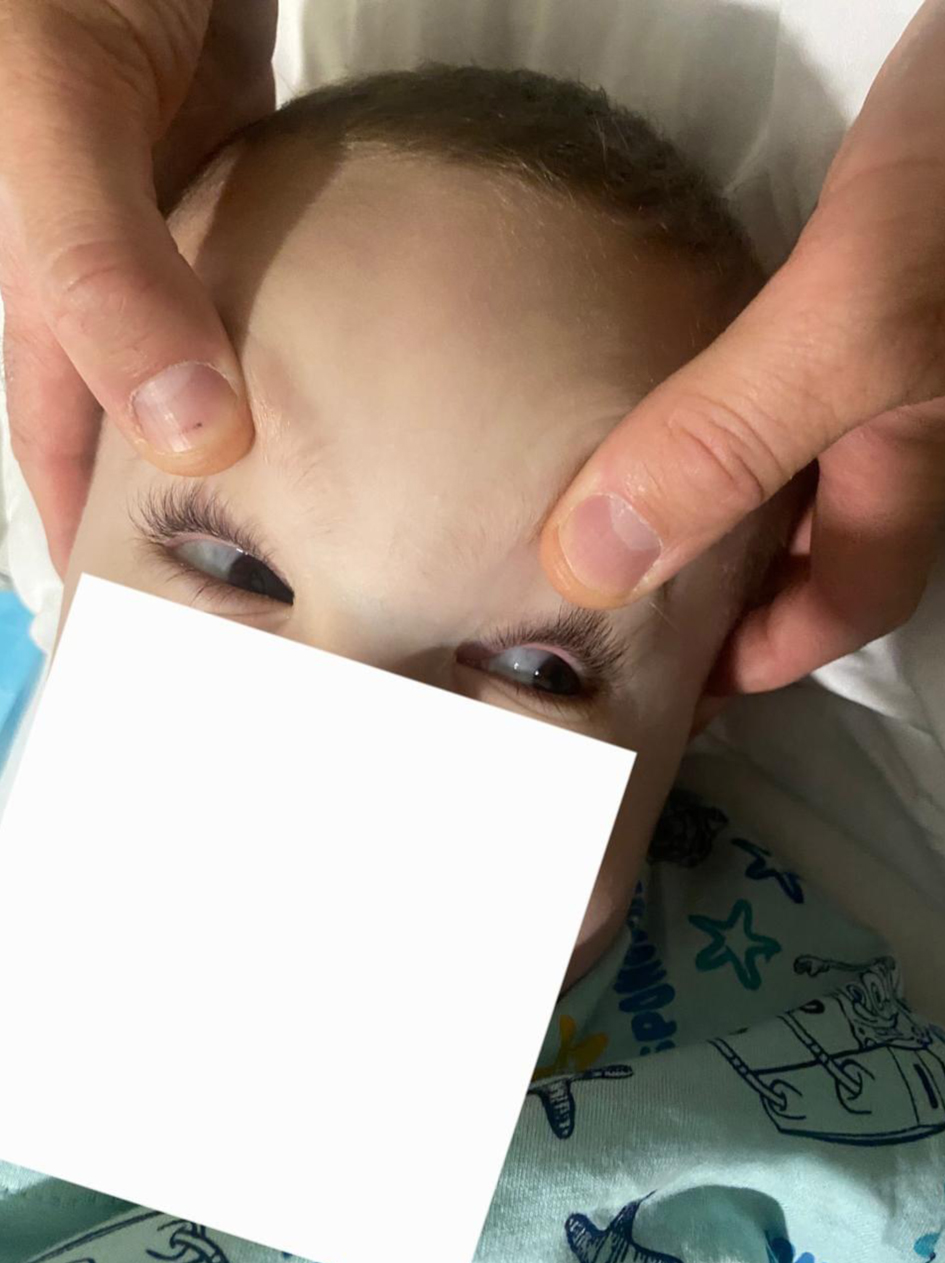 Click for large image | Figure 4. Eye deviation. |
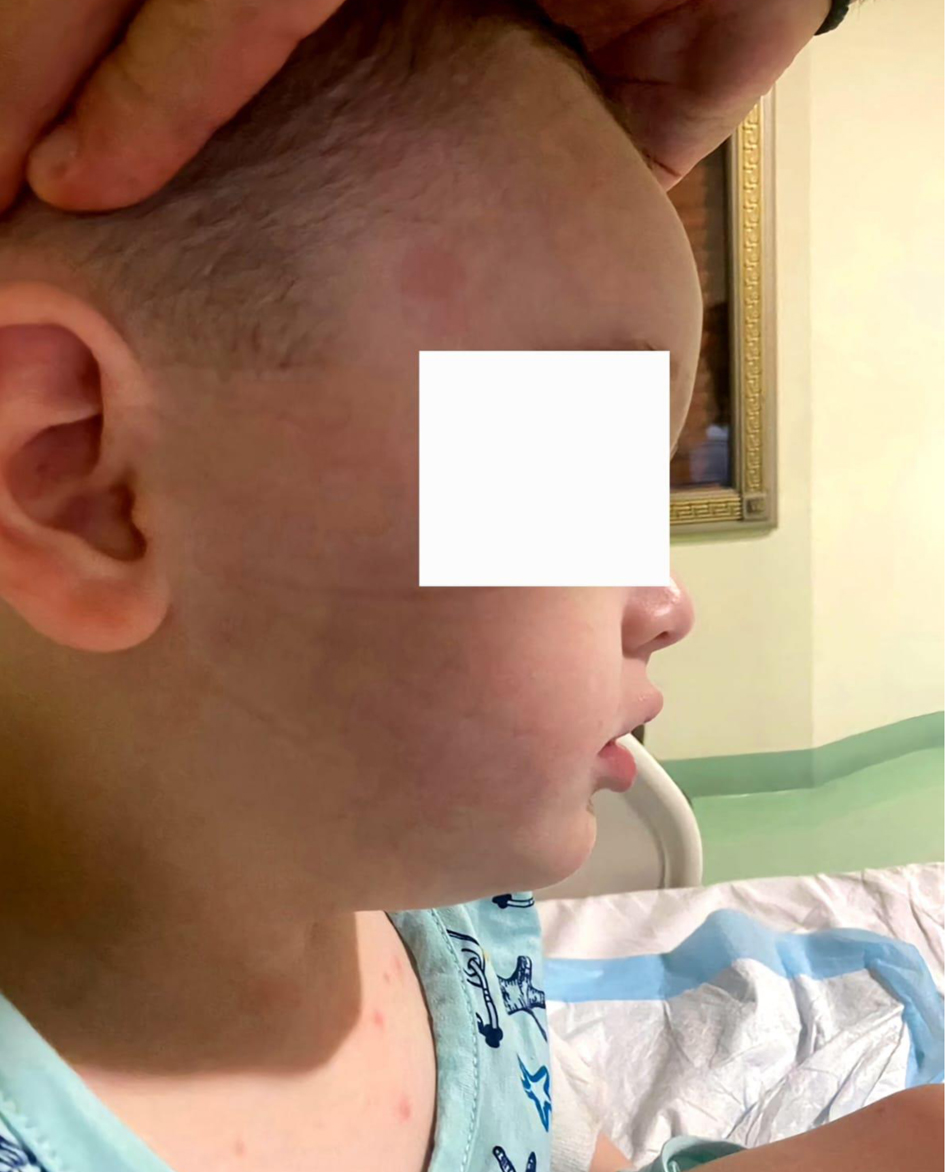 Click for large image | Figure 5. Mild micrognathia. |
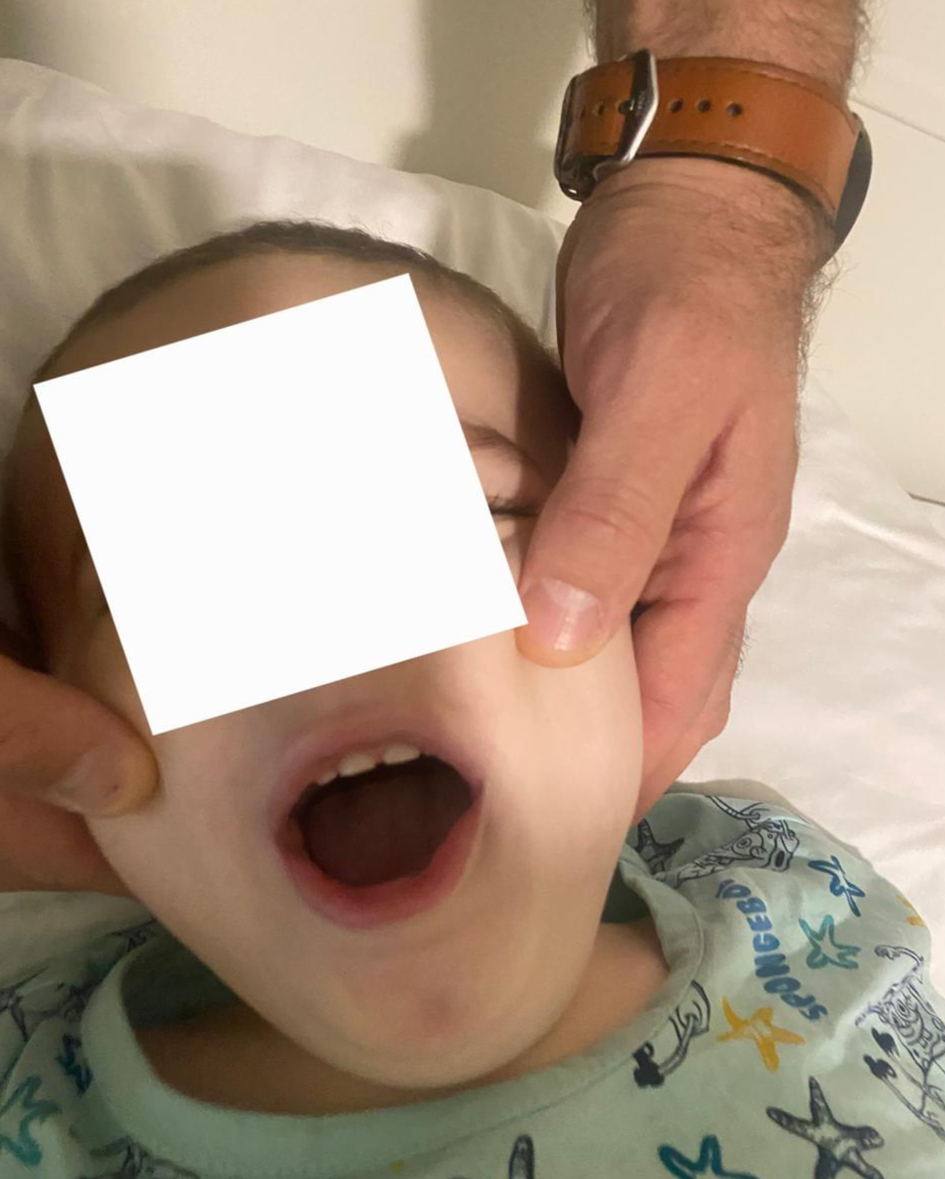 Click for large image | Figure 6. Mallampati class II-III. |
Diagnosis
No premedication was administered due to the patient’s decreased level of consciousness and the associated risk of aspiration during anesthesia induction. Standard plus intracranial pressure monitoring was applied, and a 22-gauge peripheral intravenous cannula was inserted (Figs. 7 and 8). The initial attempt at tracheal intubation using conventional laryngoscopy with a Miller blade was unsuccessful. The airway was subsequently secured using a video laryngoscope (Karl-Storz, C-MAC 8043ZX) and trachea was intubated with size 5 mm internal diameter endotracheal tube (Fig. 9).
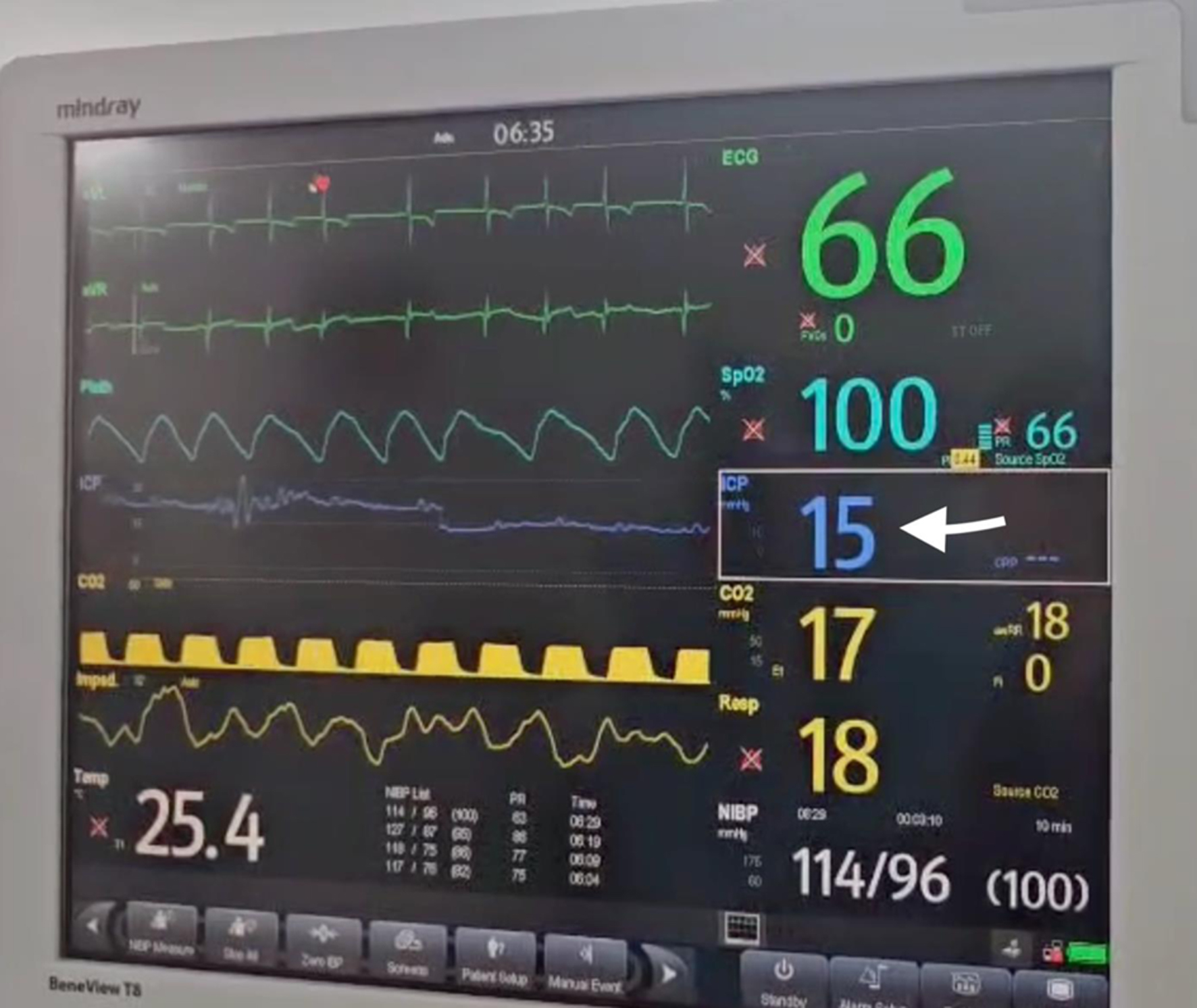 Click for large image | Figure 7. ICP monitoring (ICP was 15 mm Hg, in arrow). ICP: increased intracranial pressure. |
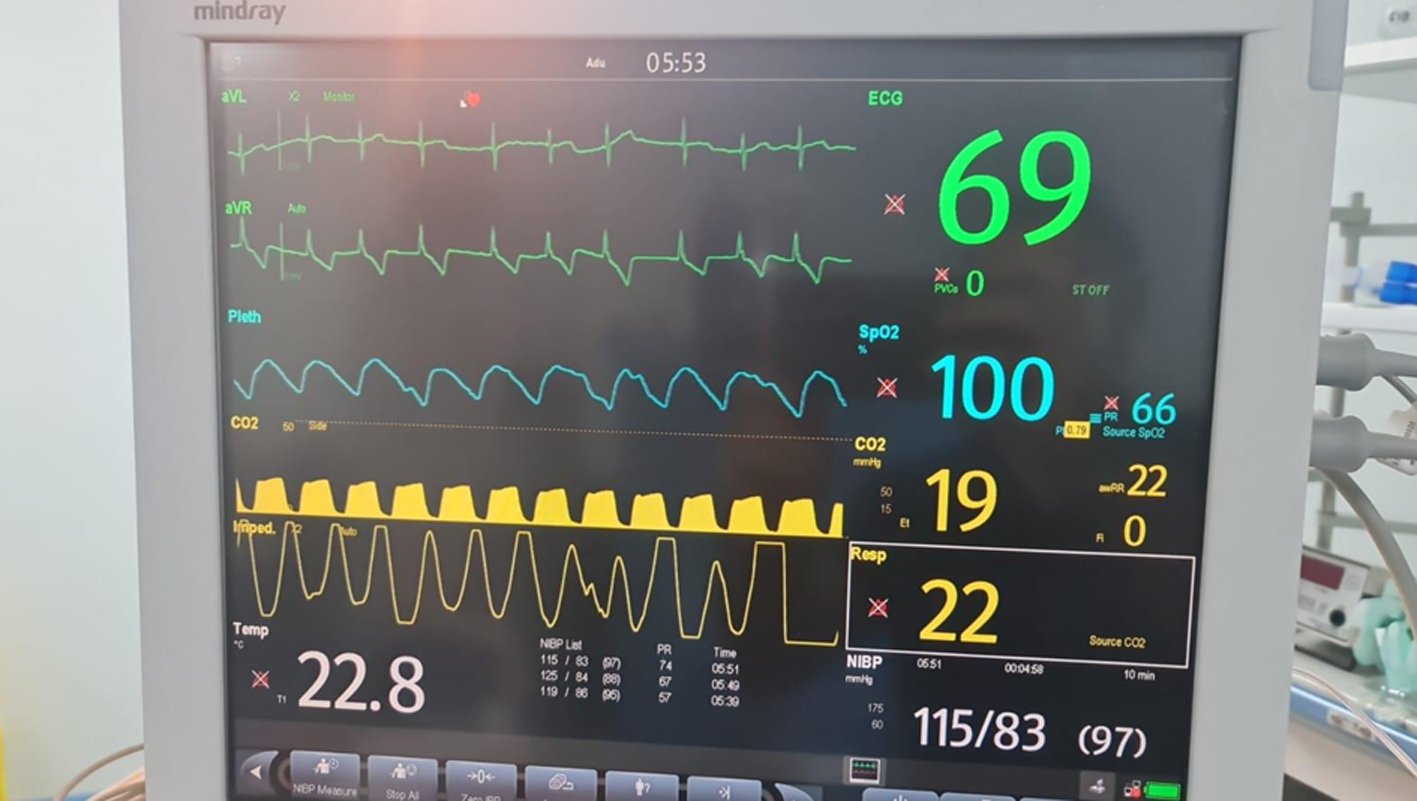 Click for large image | Figure 8. Standard monitoring. |
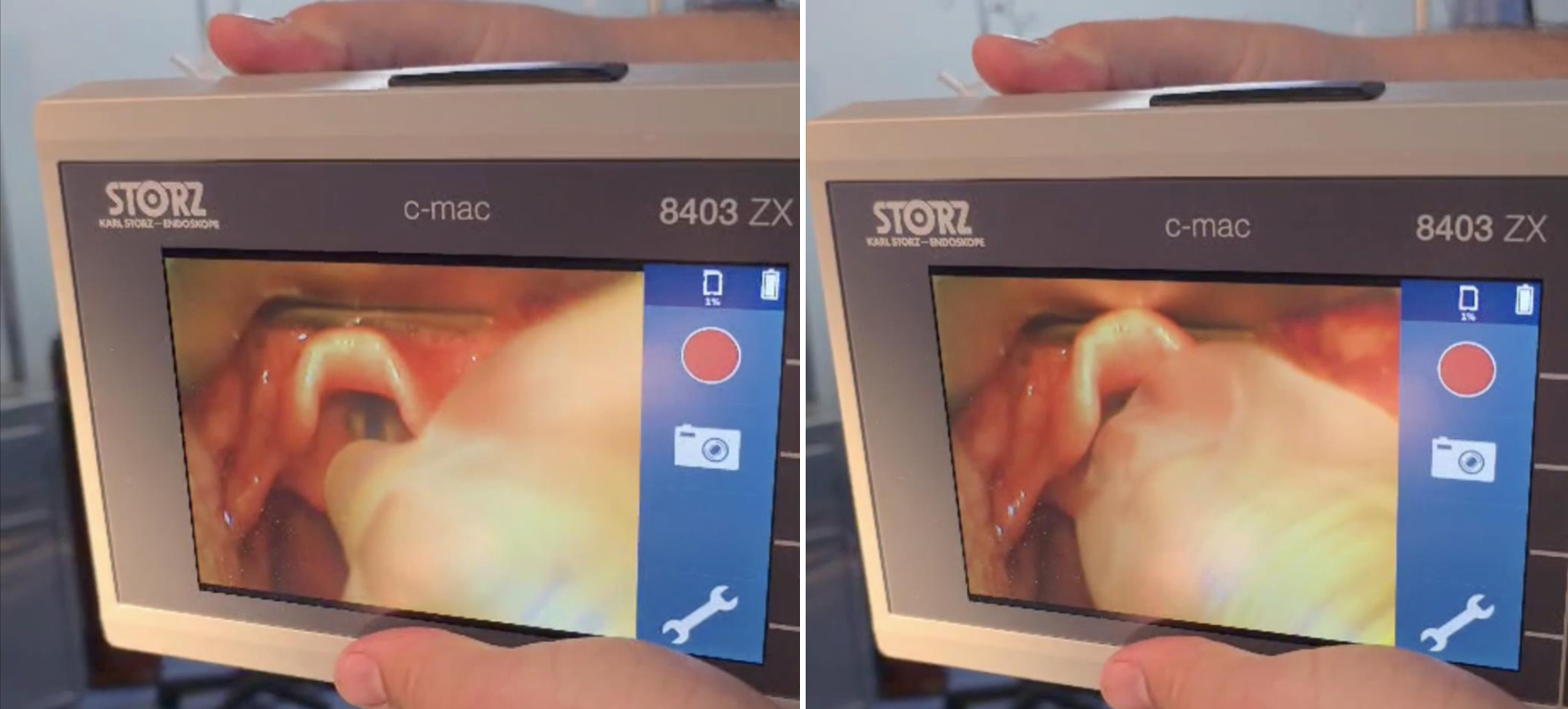 Click for large image | Figure 9. Video laryngoscopy intubation. |
Treatment
General anesthesia was induced with intravenous administration of propofol at a dose of 2 mg/kg and fentanyl at 3 µg/kg to ensure adequate hypnosis and analgesia. Anesthesia maintenance was performed using TIVA, consisting of continuous infusions of propofol at 200 µg/kg/min and remifentanil at 0.3 µg/kg/min, allowing for stable hemodynamic conditions and rapid emergence. No neuromuscular drugs were used. The surgical procedure was completed without intraoperative complications. Upon completion of surgery, the patient met extubation criteria and was successfully extubated in the operating room. No episodes of apnea or respiratory failure were observed during the 30-min observation period in the operating room following extubation.
Follow-up and outcomes
Postoperatively, the patient was transferred in stable condition to the ward for further monitoring and recovery. After postoperative imaging (head MRI) and abdominal ultrasound was performed, the patient was discharged home (Figs. 10 and 11). Informed consent was obtained from the patient’s relative, and all images are provided courtesy of the American Hospital imaging database.
 Click for large image | Figure 10. Postoperative MRI indicating shunt catheter in arrow. MRI: magnetic resonance imaging. |
 Click for large image | Figure 11. Postoperative MRI indicating reduced hydrocephaly in arrows. MRI: magnetic resonance imaging. |
| Discussion | ▴Top |
Dandy-Walker syndrome represents a complex spectrum of intracranial malformations with significant clinical and anesthetic implications. The constellation of anomalies, including hydrocephalus, cerebellar vermis hypoplasia, and cystic dilation of the fourth ventricle, presents multiple perioperative challenges. Anesthetic management requires preparedness for elevated intracranial pressure, difficult airway scenarios often compounded by craniofacial dysmorphisms, and potential postoperative respiratory complications related to brainstem dysfunction. Pediatric patients further require specialized expertise, particularly in establishing and maintaining peripheral venous access. Comprehensive preoperative assessment and multidisciplinary planning are essential to optimize outcomes [5, 6].
While principles of pediatric neurosurgical anesthesia resemble those in adults, unique challenges arise due to congenital anomalies, distinct tumor types, and variable anatomical localizations. Key considerations include monitoring and controlling intracranial pressure, managing difficult airways, securing venous access, maintaining age-appropriate mean arterial pressure, and preventing hypoxia or hypercapnia [6]. Our case involved a 3-year-old male born at term via elective cesarean section, who presented with seizures shortly after birth and was treated with valproate and levetiracetam. Neurodevelopment was nearly normal until age 2, after which progressive macrocephaly, developmental delay, recurrent pneumonia, and impaired motor function became evident as a pattern consistent with Dandy-Walker syndrome. A thorough pediatric cardiac evaluation, including examination, electrocardiography, and echocardiography, revealed no abnormalities [7].
Airway evaluation is critical in patients with craniofacial or neurodevelopmental abnormalities. Features such as macrocephaly, micrognathia, macroglossia, and cervical spine anomalies can increase the risk of difficult mask ventilation and intubation [8, 9]. In our patient, mild micrognathia and a Mallampati class II-III score were noted. Due to developmental delay and limited cooperation, comprehensive airway assessment was incomplete [9]. After induction, conventional Miller blade intubation failed, but successful endotracheal intubation was achieved with a pediatric video laryngoscope, facilitating atraumatic airway management.
Postoperative respiratory failure remains a major concern, as central nervous system (CNS) abnormalities including corpus callosum agenesis, pontine hypoplasia, and medullary respiratory center distortion may impair respiratory drive and airway reflexes [10]. Laryngospasm, which can be more frequent and severe in patients with brainstem lesions, did not occur in our patient [10]. To mitigate these risks, we used TIVA with short-acting agents, avoiding neuromuscular blocking drugs when train-of-four monitoring was unavailable. This strategy allowed rapid emergence, safe extubation, and preservation of spontaneous respiration.
Reversal of neuromuscular blockade is a critical step in anesthesia to ensure safe postoperative recovery when muscle relaxants are used. Two primary approaches, quantitative train-of-four monitoring and clinical judgment, are used to assess residual blockade. TOF monitoring provides objective, real-time data that improve the accuracy of reversal agent dosing and reduce the risk of postoperative respiratory complications [11]. Clinical judgment relies on observable patient responses and is immediately available but is subjective and less sensitive in detecting residual paralysis. Another meta-analysis of 24 trials (3,375 patients) evaluated the impact of intraoperative neuromuscular monitoring (NMM) on postoperative residual curarization (PORC). PORC incidence was lower with intermediate-acting neuromuscular blockers compared to long-acting agents. Monitoring, whether via simple peripheral nerve stimulator or objective device, did not significantly reduce PORC. Mivacurium was excluded due to limited data [12]. PORC remains common, and the effectiveness of intraoperative NMM has been unclear. This meta-analysis of 53 studies (12,664 patients) found that quantitative NMM significantly reduced PORC incidence compared to qualitative or no monitoring, particularly with intermediate-duration neuromuscular blocking agents [13]. Pediatric patients are at risk of PORC due to age-related differences in the pharmacokinetics and pharmacodynamics of neuromuscular blocking agents (NMBAs) and the short duration of many surgeries [14]. This meta-analysis of 71 trials including 4,319 participants quantified spontaneous recovery times (t25, RI25-75, tTOF90) across different NMBAs and age groups. Recovery was faster in older children compared to neonates and infants, with duration further prolonged by volatile anesthetics and aminosteroid NMBAs. The findings highlight significant variability in neuromuscular recovery, underscoring the need for quantitative NMM even after a single NMBA dose in pediatric patients [14]. The patient remained stable in the postanesthesia care unit (PACU) for 30 min with no signs of respiratory depression.
Based on this experience, ultrashort-acting agents such as propofol and remifentanil, combined with avoidance of muscle relaxants, are recommended to minimize postoperative respiratory complications [15]. Interesting findings were reported by Lee et al. In their retrospective study, volatile anesthetics showed no superiority over TIVA in reducing postoperative pulmonary complications (PPCs) after anatomical pulmonary resection in patients with lung cancer [16]. Recent evidence supports the superiority of total TIVA. In a randomized controlled trial, Chang et al compared TIVA with inhalational anesthesia, using postoperative pulmonary complications (PPCs) as the primary endpoint. Their results demonstrated that TIVA significantly reduced the incidence of PPCs [17]. Given the respective advantages of each anesthetic modality, the choice of anesthesia should be tailored to individual patient risk factors and clinical circumstances [17]. Kannabiran et al, in their narrative review, highlighted TIVA as a favorable option due to its smooth induction, faster recovery, reduced postoperative nausea and vomiting, and its capacity to optimize surgical conditions [18]. Kumaria et al similarly reported superior perioperative outcomes with TIVA, attributing the benefit largely to better intraoperative brain relaxation [19]. Oh et al published their data of a retrospective cohort study of 144,506 adults undergoing cranial neurosurgery in South Korea (2016 - 2021) compared TIVA with inhalation anesthesia. The 90-day mortality rates were similar between groups (14.0% TIVA vs. 14.2% inhalation; odds ratio (OR) 0.97, 95% confidence interval (CI) 0.94 - 1.01, P = 0.188). However, TIVA was associated with a significantly lower rate of postoperative complications (47.1% vs. 50.3%; OR 0.88, 95% CI 0.86 - 0.90, P < 0.001). Thus, while anesthetic type did not affect short-term mortality, TIVA was linked to reduced postoperative morbidity [20].
Effective postoperative pain management is essential. Opioid use may be limited due to potential respiratory depression and altered consciousness, while NSAIDs are constrained by bleeding risk. Multimodal analgesia, including regional techniques, NSAIDs, dexmedetomidine, paracetamol, ketamine, and scalp blocks, is effective for controlling postoperative pain, reserving opioids for insufficient pain control [21-24].
Generally, careful evaluation by both neurosurgeons and anesthesiologists is essential to ensure patient safety and optimize prognosis. This assessment begins with determining the indication and timing of surgery, which should be guided by an individualized approach from both surgical and anesthetic teams. In children with posterior fossa arachnoid cysts, management decisions should be based primarily on clinical presentation rather than radiological findings. Pesaresi et al found that a “wait and see” approach is effective for asymptomatic patients, while surgical decisions should consider cyst location, hydrocephalus, and patient age. Age < 12 months, hydrocephalus, macrocephaly, and shunt placement increase the risk of re-surgery. Outcomes were similar across microsurgical, endoscopic, and shunting procedures, highlighting the importance of individualized treatment planning [25].
Our anesthetic strategy, summarized in Table 1, emphasized thorough preoperative evaluation, exclusion of additional congenital anomalies, careful airway management, and prevention of postoperative respiratory complications. Difficult airway protocols were established, and postoperative respiratory care included avoidance of muscle relaxants or the use of train-of-four NMM when relaxants were necessary.
 Click to view | Table 1. Key Considerations and Recommended Actions in Pediatric Neuroanesthesia for Patients With Dandy-Walker Syndrome [7-9] |
Conclusions
Pediatric neuroanesthesia requires dual expertise in both pediatric and neuroanesthetic management. Patients with Dandy-Walker syndrome present unique perioperative challenges, necessitating comprehensive preoperative evaluation. Special attention should be paid to associated congenital anomalies, particularly cardiac malformations, airway abnormalities, and neurologically related dysfunctions. Careful airway management, including a clearly defined rescue strategy, is essential, along with the use of short-acting anesthetic agents to allow precise control of anesthesia. Muscle relaxants should be avoided if train-of-four monitoring is unavailable, and rapid emergence from anesthesia should be planned to minimize postoperative respiratory complications. The use of total intravenous anesthesia under these principles is recommended to ensure a safe and uneventful anesthetic course.
Learning points
Pediatric neuroanesthesia is a highly specialized field that demands a skilled and experienced multidisciplinary team.
Dandy-Walker syndrome poses distinct challenges, particularly concerning airway management, intracranial pressure control, and the prevention of postoperative respiratory complications.
The anesthesia plan prioritized difficult airway management, minimized pulmonary complications with short-acting intravenous agents, avoided muscle relaxants, when possible, used train-of-four monitoring if relaxants were necessary, and ensured rapid emergence.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informe consent has been obtained.
Author Contributions
RD, AA, and GH: manuscript writing; OD and FC: literature searching; RD, AD, and KL: language editing.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Khan A, Kurtz B, Ambardekar A. Lessons learned from a burn-injured pediatric patient with Dandy-Walker syndrome after multiple anesthetics: a case report. A A Pract. 2019;13(5):166-168.
doi pubmed - Banerjee Sh, Gupta N, Sarkar D, Choudhury K. Anesthetic management in an infant with Dandy-Walker syndrome presenting with acyanotic heart disease and hydrocephalous post-COVID-19 recovery: a rare experience. Journal of Neuroanaesthesiology and Critical Care. 2021;09.
doi - Datt V, Tempe DK, Lalwani P, Aggarwal S, Kumar P, Diwakar A, Tomar AS. Perioperative management of a patient with Dandy Walker malformation with tetralogy of Fallot undergoing total correction and fresh homologous pericardial pulmonary valve conduit implantation: Report of a rare case. Ann Card Anaesth. 2015;18(3):433-436.
doi pubmed - Tsirikos AI, Wordie SJ. Dandy-Walker syndrome associated with scoliosis: clinical presentation, preoperative assessment, and treatment. Case Rep Orthop. 2020;2020:8874819.
doi pubmed - Domi R. Emerging trends in paediatric neurosurgical anaesthesia: Time for subspeciality? Indian J Anaesth. 2024;68(9):750-751.
doi pubmed - Domi R, Coniglione F, Abdyli A, Huti G, Lilaj K, Bilotta F. Anaesthesia Considerations on Paediatric Neurosurgery. Turk J Anaesthesiol Reanim. 2025;53(2):34-41.
doi pubmed - Stambolliu E, Ioakeim-Ioannidou M, Kontokostas K, Dakoutrou M, Kousoulis AA. The most common comorbidities in Dandy-Walker syndrome patients: a systematic review of case reports. J Child Neurol. 2017;32(10):886-902.
doi pubmed - Moodley AW, Nel S, Oosthuizen E, Lundgren C. Anaesthetic management for ventriculoperitoneal shunt insertion in an infant with Dandy-Walker syndrome. South Afr J Anaesth Analg. 2017;23:17-20.
doi - Domi R. The best prediction test of difficult intubation. Journal of Anaesthesiology Clinical Pharmacology. 2010;26(2):193-196.
- Jang JS, Lee JJ, Park WJ, Kim EY, Lim SY. Anesthetic management of an adolescent with Dandy-Walker syndrome. Korean J Anesthesiol. 2013;64(2):180-181.
doi pubmed - Biswal D, Sahu BP, Tudu J, Das GR, Jena S, Routray D. A comparative study of train-of-four (TOF) count at corrugator supercilii and at adductor pollicis reflecting abdominal muscles relaxation. International Journal of Health Sciences. 2022;6(S6):3816-3824.
- Naguib M, Kopman AF, Ensor JE. Neuromuscular monitoring and postoperative residual curarisation: a meta-analysis. Br J Anaesth. 2007;98(3):302-316.
doi pubmed - Carvalho H, Verdonck M, Cools W, Geerts L, Forget P, Poelaert J. Forty years of neuromuscular monitoring and postoperative residual curarisation: a meta-analysis and evaluation of confidence in network meta-analysis. Br J Anaesth. 2020;125(4):466-482.
doi pubmed - Vanlinthout LE, Driessen JJ, Stolker RJ, Lesaffre EM, Berghmans JM, Staals LM. Spontaneous recovery from neuromuscular block after a single dose of a muscle relaxant in pediatric patients: A systematic review using a network meta-analytic and meta-regression approach. Paediatr Anaesth. 2024;34(8):720-733.
doi pubmed - Domi R. Total intravenous anaesthesia in neurosurgery: the fading role of inhalation agents? Indian J Anaesth. 2025;69(5):428-431.
doi pubmed - Lee S, Cho JS, Kim E, Kim Y, Lee J. Effects of inhalation versus total intravenous anesthesia on postoperative pulmonary complications after anatomic pulmonary resection. J Chest Surg. 2022;55(1):30-36.
doi pubmed - Chang YT, Lai CS, Lu CT, Wu CY, Shen CH. Effect of total intravenous anesthesia on postoperative pulmonary complications in patients undergoing microvascular reconstruction for head and neck cancer: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2022;148(11):1013-1021.
doi pubmed - Kannabiran N, Bidkar P. Total intravenous anesthesia for neurosurgery. J Neuroanesthesiol Crit Care. 2018;5:141-149.
- Kumaria A, Hughes M, Fenner H, Moppett IK, Smith SJ. Total intravenous anaesthesia with propofol and remifentanil is associated with reduction in operative time in surgery for glioblastoma when compared with inhalational anaesthesia with sevoflurane. J Clin Neurosci. 2024;120:191-195.
doi pubmed - Oh TK, Song IA, Jeon YT. Comparison of postoperative outcomes after cranial neurosurgery using propofol-based total intravenous anesthesia versus inhalation anesthesia: a nationwide cohort study in South Korea. Korean J Anesthesiol. 2024;77(6):614-622.
doi pubmed - Kulikov A, Tere V, Sergi PG, Bilotta F. Prevention and treatment of postoperative pain in pediatric patients undergone craniotomy: Systematic review of clinical evidence. Clin Neurol Neurosurg. 2021;205:106627.
doi pubmed - Mestdagh FP, Lavand'homme PM, Pirard G, Joshi GP, Sauter AR, Van de Velde M, Anaesthesia PWGotESoR, et al. Pain management after elective craniotomy: a systematic review with procedure-specific postoperative pain management (PROSPECT) recommendations. Eur J Anaesthesiol. 2023;40(10):747-757.
doi pubmed - Domi R, Cani A, Abdyli A, Huti G, Dodaj S, Coniglione F, Grada M, et al. Ketamine in neurocritical care: new potentials and perspectives. Cureus. 2025;17(6):e85456.
doi pubmed - Festa R, Tosi F, Pusateri A, Mensi S, Garra R, Mancino A, Frassanito P, et al. The scalp block for postoperative pain control in craniosynostosis surgery: a case control study. Childs Nerv Syst. 2020;36(12):3063-3070.
doi pubmed - Pesaresi A, Piatelli G, Garbossa D, Pavanello M. Posterior Fossa Arachnoid cysts (PFACs) in pediatric patients: a single-center retrospective study and proposal of a treatment flow-chart. Acta Neurochir (Wien). 2024;166(1):428.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.