| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com |
Case Report
Volume 000, Number 000, January 2025, pages 000-000
When the Tumor Leaves but the Damage Lingers: A Case of Delayed Cardiomyopathy Recovery Post-Paraganglioma Resection
Josephine Ria Pitasaria, c, Sajjad Gulb, Jerry Kenmoea, Ahmad Munirb
aDepartment of Internal Medicine, McLaren Health Care/Michigan State University, Flint, MI, USA
bDepartment of Cardiology, McLaren Health Care/Michigan State University, Flint, MI, USA
cCorresponding Author: Josephine Ria Pitasari, Department of Internal Medicine, McLaren Health Care/Michigan State University, Flint, MI, USA
Manuscript submitted July 7, 2025, accepted July 31, 2025, published online January xx, 2025
Short title: Cardiomyopathy Post-Paraganglioma Resection
doi: https://doi.org/10.14740/jmc5166
| Abstract | ▴Top |
Catecholamine-induced cardiomyopathy secondary to paraganglioma is a rare and potentially reversible condition. However, the course of recovery post-resection remains variable and may be delayed despite biochemical cure. We present the case of a 47-year-old male with biopsy-confirmed extra-adrenal paraganglioma who developed acute decompensated heart failure due to catecholamine-induced cardiomyopathy (left ventricular ejection fraction (LVEF) 30-35%) and multiorgan dysfunction. Despite successful surgical resection, his LVEF remained reduced postoperatively, and he was discharged on heart failure therapy and a wearable cardioverter defibrillator. A follow-up echocardiogram showed improvement in LVEF to 45% 2 weeks later, but his blood pressure remained poorly controlled despite adherence to a multi-drug regimen and lifestyle measures. He was readmitted with a transient ischemic attack (TIA) shortly after surgery. This case illustrates the variable recovery trajectory in paraganglioma-induced cardiomyopathy and highlights persistent cardiovascular risks, including resistant hypertension and cerebrovascular events despite biochemical cure. It emphasizes the importance of ongoing cardiac surveillance and multidisciplinary management, particularly in patients facing socioeconomic barriers to follow-up care.
Keywords: Cardiomyopathy, Paraganglioma; Pheochromocytoma; Catecholamine; Hypertension
| Introduction | ▴Top |
Pheochromocytomas and paragangliomas (PPGLs) are rare neuroendocrine tumors that arise from chromaffin cells and secrete catecholamines. Pheochromocytomas are estimated to occur in approximately 0.05% to 0.2% of hypertensive individuals. While traditionally it was believed that 10% of these tumors are extra-adrenal, recent data suggest that about 15-20% of pheochromocytomas are extra-adrenal paragangliomas in adults, with higher percentages in pediatric populations. These extra-adrenal tumors most commonly originate in the retroperitoneum along the sympathetic chain [1]. Excess catecholamine release from these tumors can precipitate hypertensive emergencies and lead to cardiovascular complications, including catecholamine-induced cardiomyopathy. While this form of cardiomyopathy is often reversible following tumor resection, the timeline for recovery can vary, and in some cases, cardiac dysfunction may persist. Early diagnosis and intervention are critical to prevent irreversible myocardial damage [2]. In this case report, we present a patient with delayed myocardial recovery and persistent hypertension following surgical resection of a paraganglioma.
| Case Report | ▴Top |
Investigations
A 47-year-old male presented with a hypertensive emergency and symptoms of headache, palpitations, and diaphoresis in September 2024. His history included hypertension, and workup revealed elevated normetanephrine with normal metanephrine levels. A preoperative electrocardiogram (ECG) showed sinus tachycardia with nonspecific ST-T changes. He was later discharged after resolution of hypertensive emergency. A biopsy done at admission later confirmed a neuroendocrine tumor consistent with extra-adrenal paraganglioma. He was later readmitted to the hospital with similar symptoms. He developed multiorgan involvement including acute kidney injury, type II non-ST-segment elevation myocardial infarction (NSTEMI), thrombocytopenia, and hemolytic anemia. His previous echocardiogram in June 2024 showed left ventricular ejection fraction (LVEF) 45-50%, which declined to 30-35% by November 2024. A computed tomography (CT) scan showed a 14-cm paraganglioma encasing both renal arteries, renal veins and inferior vena cava (IVC). He shortly underwent preoperative stabilization with alpha and beta blockade.
Diagnosis
Diagnosis was confirmed by histopathology after resection on November 27, 2024. Immunohistochemistry was positive for chromogranin A, GATA3, and S100. Intraoperatively, the tumor was found located in the right retroperitoneum, extending anterior and posterior to the inferior vena cava and encasing right renal vasculature, consistent with a sympathetic paraganglioma arising from the interaortocaval or paracaval region. Initial differential diagnoses included hypertensive heart disease and restrictive cardiomyopathy, which were ruled out based on echocardiogram and magnetic resonance imaging (MRI) findings. His echocardiogram showed LVEF of 30% with moderate left ventricular hypertrophy (LVH) and global left ventricular (LV) hypokinesis, suggesting catecholamine-induced cardiomyopathy in light of his biopsy-proven paraganglioma. Endocrinology was consulted perioperatively for optimization of catecholamine blockade.
Treatment
Initial management included phentolamine, phenoxybenzamine, doxazosin, carvedilol, and nifedipine. Most agents were administered orally, with phentolamine given intravenous (IV) during hypertensive crisis. Phenoxybenzamine and doxazosin were long-acting oral agents used preoperatively, while carvedilol and nifedipine extended release (ER) were also oral formulations for blood pressure (BP) control. Postoperatively, he was placed on nifedipine ER, carvedilol, prazosin, and losartan. He was also started on dual antiplatelet therapy and high-intensity statin after transient ischemic attack (TIA) in December 2024. He was placed on a LifeVest due to reduced LVEF and was closely monitored in outpatient follow-up.
Follow-up and outcomes
Postoperative normetanephrine levels declined markedly from > 50 nmol/L to 9.24 nmol/L, indicating substantial biochemical improvement and effective tumor debulking. However, the fragmented pathology specimen with unassessable margins, along with focal capsular and lymphovascular invasion, precludes confirmation of complete resection. Continued biochemical and radiologic surveillance is warranted but was limited by the patient’s socioeconomic barriers. On December 12, 2024, he was readmitted with a TIA. MRI showed no infarct; bubble study was negative. Echo showed improved LVEF (45%). Despite adherence to medications and lifestyle, BP remained poorly controlled (up to 201/153 mm Hg). Clinic follow-ups in early 2025 confirmed resistant hypertension. Nifedipine was increased, and spironolactone was considered. He remained asymptomatic from a heart failure standpoint and off LifeVest. Genetic testing for hereditary paraganglioma syndromes (i.e., multiple endocrine neoplasia (MEN)) was recommended, though not completed due to financial constraints.
| Discussion | ▴Top |
Paraganglioma-induced cardiomyopathy is an uncommon but serious manifestation of catecholamine-secreting tumors. These tumors, which arise from extra-adrenal chromaffin tissue, can cause profound cardiovascular effects due to excess catecholamine secretion - particularly norepinephrine - resulting in sustained hypertension, vasoconstriction, and direct myocardial toxicity [2, 3]. The pathophysiology involves a multifactorial cascade, including oxidative stress, calcium overload, coronary vasospasm, and chronic beta-adrenergic receptor stimulation, which collectively lead to myocardial necrosis, fibrosis, and remodeling [4]. These effects can culminate in either a Takotsubo-like reversible cardiomyopathy or a dilated phenotype, with variable recovery depending on the chronicity of catecholamine exposure and individual patient vulnerability [5].
The imaging findings in this case support the diagnosis of catecholamine-induced cardiomyopathy secondary to pheochromocytoma that was initially diagnosed with biopsy. On admission, transthoracic echocardiography demonstrated a markedly reduced ejection fraction with LVEF 30-35% with global hypokinesis, a pattern that has been described in patients with acute catecholamine excess. CT of the abdomen revealed a hyper-enhancing retroperitoneal mass (Fig. 1), which was later confirmed to be a paraganglioma on histopathology (Fig. 2). Notably, repeat echocardiography following surgical resection of the tumor showed some improvement in wall motion abnormalities but persistent systolic dysfunction (Fig. 3) ( Supplementary Material 1, jmc.elmerpub.com), suggesting a degree of myocardial stunning or irreversible injury. These imaging findings emphasize the dynamic yet potentially incomplete reversibility of cardiac dysfunction in pheochromocytoma-associated cardiomyopathy and highlight the importance of early recognition and management of the underlying tumor. Cardiac MRI was not performed due to institutional limitations. Likewise, a right heart catheterization was not pursued by the managing cardiology team, given the patient’s clinical improvement prior to discharge.
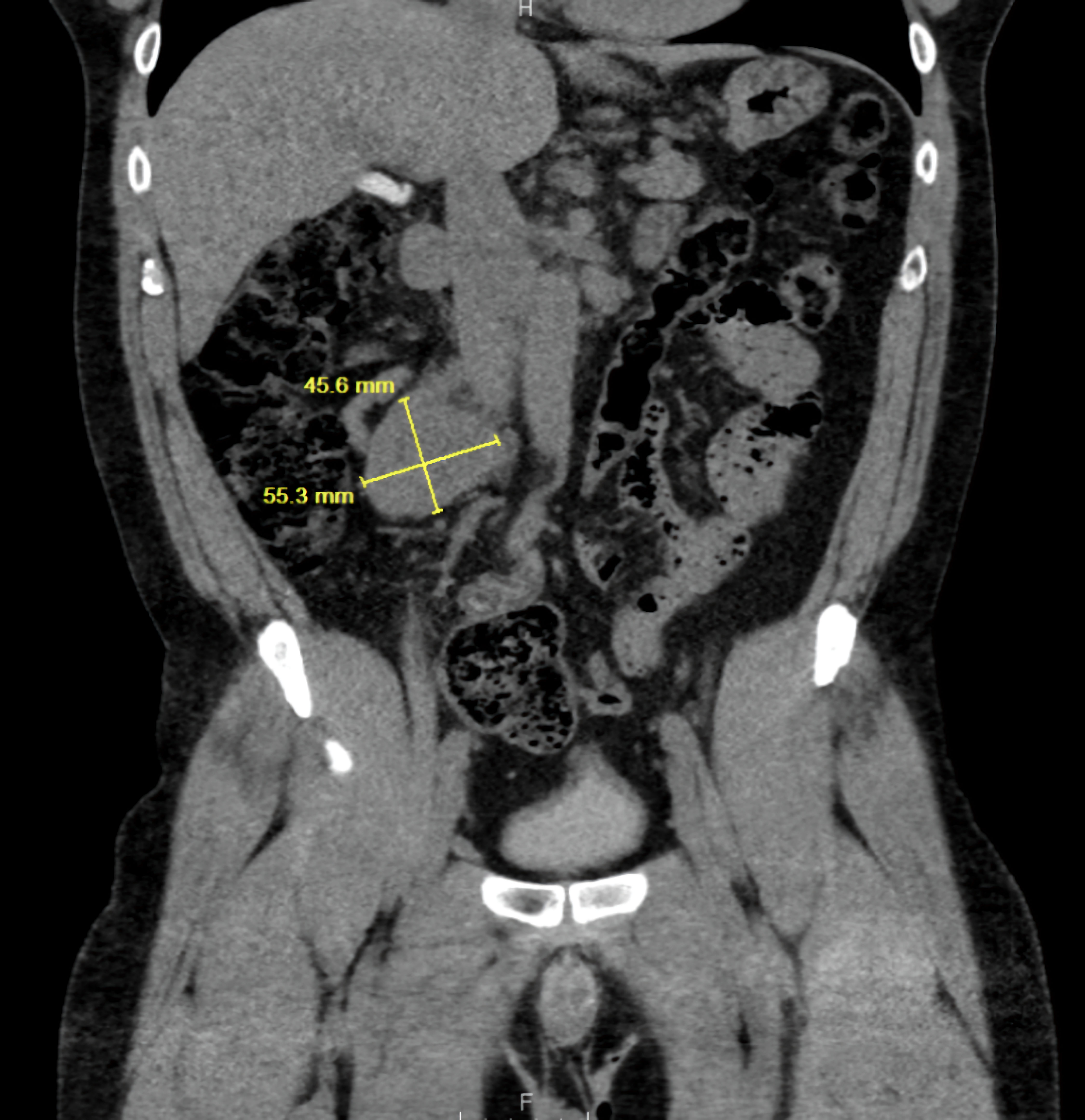 Click for large image | Figure 1. Coronal CT abdomen showing a retroperitoneal mass measuring 55.3 × 45.0 mm (yellow measurement line), consistent with an extra-adrenal paraganglioma. CT: computed tomography. |
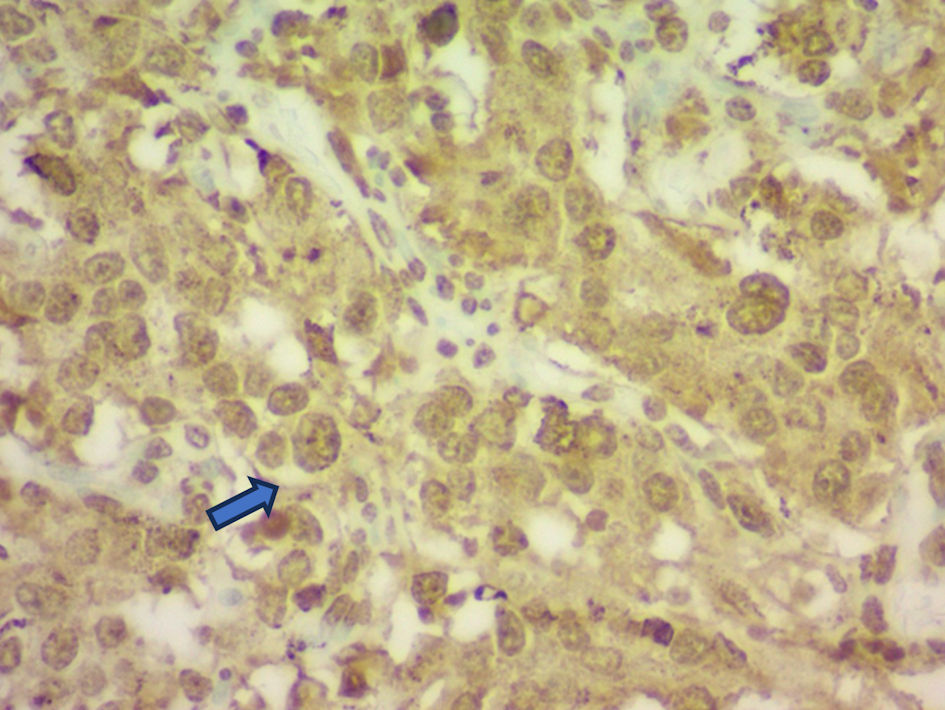 Click for large image | Figure 2. Histopathology of the resected paraganglioma demonstrating neuroendocrine cell morphology (blue arrow) with positive chromogranin A immunostaining. |
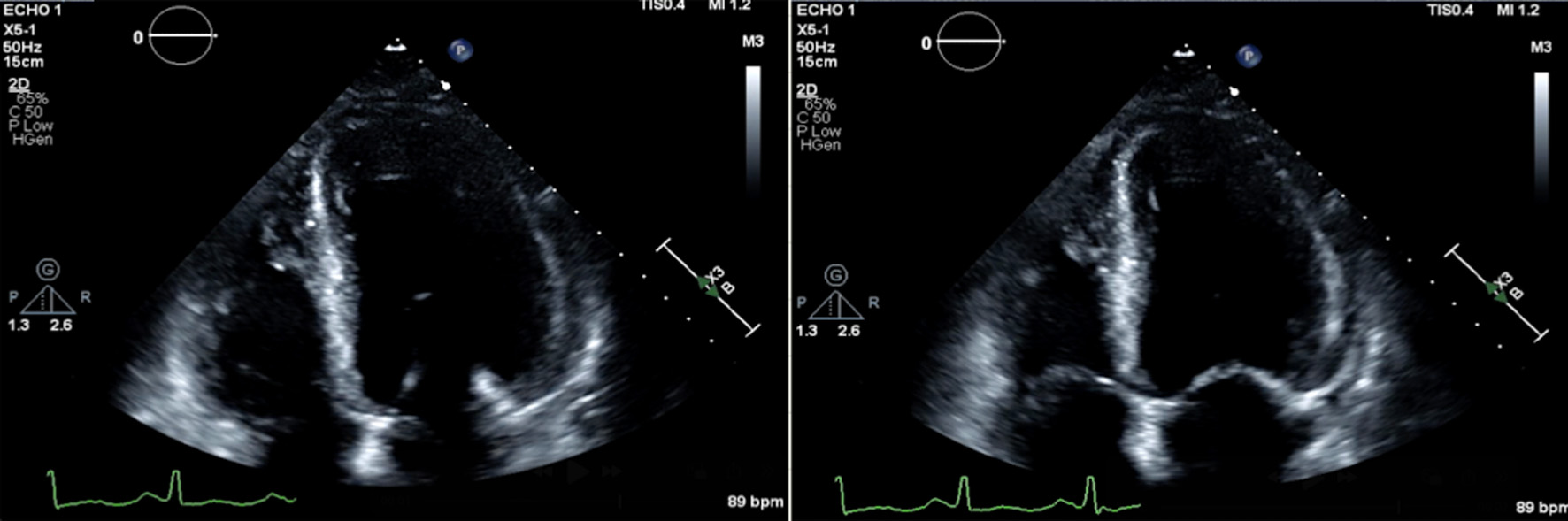 Click for large image | Figure 3. Apical four-chamber views of echocardiographic findings demonstrating reduced left ventricular systolic function, obtained six days post-procedure with an estimated ejection fraction (EF) of 30%. The left image shows the heart in end-diastole, with the left ventricle at maximal volume. The right image shows end-systole, demonstrating poor ventricular contraction with minimal reduction in LV cavity size. |
While many patients experience rapid cardiac improvement after tumor resection, complete recovery is not universal. Chanthar et al reported that although the majority of patients normalize their ejection fraction (EF) within 4 - 12 weeks postoperatively, delayed or incomplete recovery may occur in those with longer preoperative catecholamine exposure or irreversible structural damage [6]. In our case, the patient had a baseline EF of 45-50% in June, which declined to 30-35% in November - coinciding with peak clinical deterioration - and improved modestly to 40-45% by mid-December after tumor resection. While we cannot definitively characterize this as delayed recovery due to the limited interval between postoperative imaging studies, the timeline suggests partial and possibly protracted myocardial healing, consistent with previously described delayed remodeling following catecholamine withdrawal [7].
The cardiovascular burden in this case extended beyond systolic dysfunction. The patient experienced TIA shortly after discharge, raising concern for ongoing vascular injury and endothelial dysfunction despite surgical cure. This is supported by literature suggesting that catecholamine excess may promote platelet activation, vasospasm, and a prothrombotic state, potentially persisting even after resection [8]. Imaging (including CT abdomen/pelvis showing a 14-cm paraganglioma encasing both renal arteries) and histopathology (with chromogranin A, GATA3, and S100 positivity) confirmed the tumor burden and support the diagnosis.
Another compelling aspect of this case is the persistence of uncontrolled hypertension postoperatively. While our center is not a designated paraganglioma center, urgent in-house surgical treatment was pursued due to the patient’s rapid clinical deterioration and that limited safe transfer. Although BP is expected to normalize after paraganglioma resection, up to 50% of patients may remain hypertensive due to underlying essential hypertension, structural vascular changes, or coexisting secondary causes such as primary aldosteronism or obstructive sleep apnea [9, 10].
The patient was managed with carvedilol and losartan for his new onset heart failure with reduced ejection fraction (HFrEF). Spironolactone was considered but deferred initially. Entresto and sodium-glucose cotransporter 2 (SGLT2) inhibitors were not started due to impaired renal function and formulary restrictions. The plan was to optimize guideline-directed medical therapy (GDMT) in the outpatient setting; however, the patient did not follow up with cardiology and was instead seen at a free clinic with limited resources. Socioeconomic barriers further contributed to delays in escalating goal-directed medical therapy. Follow-up clinic notes reveal continued systolic readings > 180 mm Hg despite intensive pharmacologic and lifestyle therapy, and socioeconomic limitations may have contributed to suboptimal management.
This case underscores the importance of serial echocardiographic monitoring, as well as aggressive risk factor modification even after biochemical cure. The anticipated inclusion of echocardiographic images pre- and post-resection, CT findings demonstrating tumor location and vascular involvement, and histology slides will further strengthen the educational and diagnostic value of this report. It also emphasizes the critical role of multidisciplinary care - involving endocrinology, cardiology, surgery, and primary care - in managing both the acute and chronic sequelae of paraganglioma-induced cardiomyopathy.
This case also raises the importance of evaluating the interplay between cardiac and neurologic complications in the postoperative period. Although rare, TIA following paraganglioma resection may reflect residual autonomic instability or endothelial dysfunction driven by previous catecholamine excess. A more comprehensive workup, including ambulatory BP monitoring and advanced vascular imaging, may be warranted in similar patients. Furthermore, public health disparities should be addressed in future studies to assess their influence on outcomes in rare endocrine-cardiovascular overlap disorders.
Learning points
Paraganglioma-induced cardiomyopathy may not always resolve immediately after tumor resection. Delayed recovery of systolic function is possible even after successful removal of catecholamine-producing tumors. Persistent hypertension post-paraganglioma is often multifactorial and may reflect irreversible vascular remodeling. Additionally, socioeconomic barriers such as limited access to specialty care and essential medications can significantly hinder recovery. Close cardiac monitoring and a multidisciplinary approach are essential to optimize long-term cardiovascular outcomes, particularly in patients with delayed diagnosis, prolonged catecholamine exposure, or limited resources.
| Supplementary Material | ▴Top |
Suppl 1. Apical four-chamber view on transthoracic echocardiography demonstrating global hypokinesis with LVEF remained estimated at 30-35%, 6 days postoperatively.
Acknowledgments
The authors would like to thank the multidisciplinary care team involved in the patient’s management during his hospital stay, including cardiology, endocrinology, and critical care service.
Financial Disclosure
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare that they have no conflict of interest related to this case report.
Informed Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Author Contributions
Josephine Ria Pitasari, MD: primary author, manuscript drafting, and clinical documentation. Sajjad Gul, MD: case conceptualization, editing, and clinical input. Jerry Kenmoe, MD: manuscript revision. Ahmad Munir, MD: supervision, final review, and clinical oversight. All authors read and approved the final version of the manuscript.
Data Availability
All data supporting the findings of this case report are included within the manuscript. Additional information is available from the corresponding author upon reasonable request.
| References | ▴Top |
- Lenders JWM, Kerstens MN, Amar L, Prejbisz A, Robledo M, Taieb D, Pacak K. Genetics, diagnosis, and management of pheochromocytoma and paraganglioma in 2022: A position statement and update of the 2014 Endocrine Society clinical practice guideline. Eur J Endocrinol. 2022;186(5):R197-R211.
doi - Zhang R, Gupta D, Albert SG. Pheochromocytoma as a reversible cause of cardiomyopathy: Analysis and review of the literature. Int J Cardiol. 2017;249:319-323.
- Prejbisz A, Lenders JW, Eisenhofer G, Januszewicz A. Cardiovascular manifestations of phaeochromocytoma. J Hypertens. 2011;29(11):2049-2060.
doi pubmed - Giavarini A, Chedid A, Bobrie G, Plouin PF, Hagege A, Amar L. Acute catecholamine cardiomyopathy in patients with phaeochromocytoma or functional paraganglioma. Heart. 2013;99(19):1438-1444.
doi pubmed - Agarwal V, Kant G, Hans N, Messerli FH. Takotsubo-like cardiomyopathy in pheochromocytoma. Int J Cardiol. 2011;153(3):241-248.
doi pubmed - Chanthar V, Khanna R, Agarwal G, et al. Cardiac changes and their reversal following curative surgery in pheochromocytoma and paraganglioma: A prospective cohort study. World J Surg. 2022;46(5):1123-1131.
- Koulouris S, Pastromas S, Sakellariou D, et al. Recovery of myocardial function after pheochromocytoma resection: a serial echocardiographic and tissue Doppler study. Echocardiography. 2009;26(3):226-231.
- Wu H, Zhang R, Jiang Y, et al. Reversible cardiomyopathy and cerebrovascular events in pheochromocytoma: A retrospective study of 108 cases. Clin Cardiol. 2018;41(10):1272-1278.
- Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942.
doi pubmed - Goh SY, Tan MZ, Liew HJ, Tan SY. Persistent hypertension after adrenalectomy for pheochromocytoma: A review of predictive factors. Clin Hypertens. 2021;27:15.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.