| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com |
Case Report
Volume 16, Number 9, September 2025, pages 372-380
Acute Hepatitis in a Patient Treated With Ribociclib for Metastatic Breast Carcinoma
Chika Iguha, b, Iqra Bakhsha, Sava Grujica
aDepartment of Pathology and Laboratory Medicine, Harbor-UCLA Medical Center, Torrance, CA 90502, USA
bCorresponding Author: Chika Iguh, Department of Pathology and Laboratory Medicine, Harbor-UCLA Medical Center, Torrance, CA 90502, USA
Manuscript submitted July 2, 2025, accepted September 5, 2025, published online September 17, 2025
Short title: Ribociclib Induced Liver Injury
doi: https://doi.org/10.14740/jmc5163
| Abstract | ▴Top |
Ribociclib, a cyclin-dependent kinase 4/6 (CDK4/6) inhibitor, is widely used in the treatment of hormone receptor-positive (HR+), human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer. Although hepatotoxicity is a recognized adverse effect, severe cases of ribociclib-induced liver injury with histologic confirmation of submassive hepatic necrosis remain rare. We describe a case of a postmenopausal woman with newly diagnosed stage IV HR+/HER2-negative invasive lobular carcinoma who developed acute hepatocellular injury 8 weeks after initiating ribociclib and anastrozole. The patient presented with fatigue, jaundice, and dark urine, and was found to have markedly elevated transaminases (alanine aminotransferase 1,825 U/L; aspartate aminotransferase 1,536 U/L) and hyperbilirubinemia. A thorough workup excluded viral, autoimmune, and obstructive hepatobiliary causes. Liver biopsy demonstrated confluent centrilobular necrosis without fibrosis or significant inflammation. Causality assessments yielded an R-factor of 20.73, a Roussel Uclaf Causality Assessment Method score of 10 (highly probable), and a Naranjo score of 7 (probable). Ribociclib was discontinued and intravenous N-acetylcysteine (NAC) initiated, leading to gradual normalization of liver enzymes. The patient was maintained on anastrozole alone, with no recurrence of liver injury and stable disease at 13-month follow-up. This case highlights the potential for ribociclib to induce severe hepatocellular injury with histologic evidence of submassive necrosis. Early recognition and structured causality assessment ensures patient safety. In patients with significant hepatotoxicity, discontinuation of ribociclib and non-rechallenge may be prudent. Furthermore, consideration of NAC in management is important in cases demonstrating persistent transaminitis despite ribociclib discontinuation.
Keywords: Ribociclib; Drug-induced liver injury; Hepatotoxicity; Cyclin-dependent kinase 4 and 6 inhibitors; Breast neoplasms; Lobular carcinoma; N-acetylcysteine; Liver biopsy
| Introduction | ▴Top |
Ribociclib, a selective cyclin-dependent kinase 4/6 (CDK4/6) inhibitor, has emerged as a cornerstone therapy in the management of hormone receptor-positive (HR+), human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer. By inhibiting the cyclin D-CDK4/6-Rb axis, ribociclib arrests tumor cell proliferation at the G1-to-S phase transition, significantly improving progression-free survival when combined with endocrine therapy, as demonstrated in the MONALEESA clinical trials [1-3]. While generally well-tolerated, ribociclib is associated with a range of adverse events, including hematologic toxicities, QT prolongation, and notably, hepatotoxicity [1-3].
Among the CDK4/6 inhibitors, ribociclib carries a relatively higher risk of hepatic injury, with grade 3-4 alanine aminotransferase (ALT) elevations reported in up to 9.3% of patients and aspartate aminotransferase (AST) elevations in up to 6.5%, typically arising within the first three treatment cycles [1-3]. Although these liver enzyme elevations are often asymptomatic and reversible with dose adjustment or discontinuation, cases of severe drug-induced liver injury (DILI) have been reported, very few showing features of submassive hepatic necrosis. Given the increasing use of CDK4/6 inhibitors in clinical oncology, heightened awareness and systematic evaluation of DILI are essential for early recognition and intervention.
We present a case of ribociclib-induced hepatocellular injury manifesting as acute hepatitis with histologically confirmed confluent centrilobular necrosis. This case contributes to the growing body of literature documenting severe hepatotoxicity associated with ribociclib and emphasizes the value of integrating structured causality assessment tools, such as the R-factor, Roussel Uclaf Causality Assessment Method (RUCAM) score, and Naranjo algorithm, with histopathologic correlation to support diagnosis and guide management.
| Case Report | ▴Top |
Clinical presentation, imaging, and pathologic workup
A postmenopausal woman with a history of diabetes mellitus, hypothyroidism, hypertension, major depression, and osteoarthritis presented to our hospital in January 2024 following a fall, with complaints of acute onset dyspnea and 6 months of progressive left-sided rib pain which she initially attributed to musculoskeletal causes. Chest X-ray showed multiple rib fractures with deformities and bilateral lower lobe opacities. A computed tomography (CT) scan of the abdomen and pelvis (Fig. 1a, b) demonstrated extensive osteolytic lesions throughout the ribs, spine, and pelvis, along with a stable 1.6 cm left adrenal nodule likely representing a benign adenoma. Magnetic resonance imaging (MRI) of the cervical and thoracic spine confirmed multifocal osteolytic lesions (C3-T1), raising suspicion for metastatic disease, without evidence of metastatic involvement in solid organs. Positron emission tomography (PET)/CT showed multiple lytic lesions involving the spine, several ribs, and the pelvis with mild FDG uptake (maximum standardized uptake value (SUVmax) 4.7) as well as an enlarged left axillary lymph node with minimal FDG uptake (SUVmax 1.5) and the aforementioned left adrenal nodule with no significant FDG uptake.
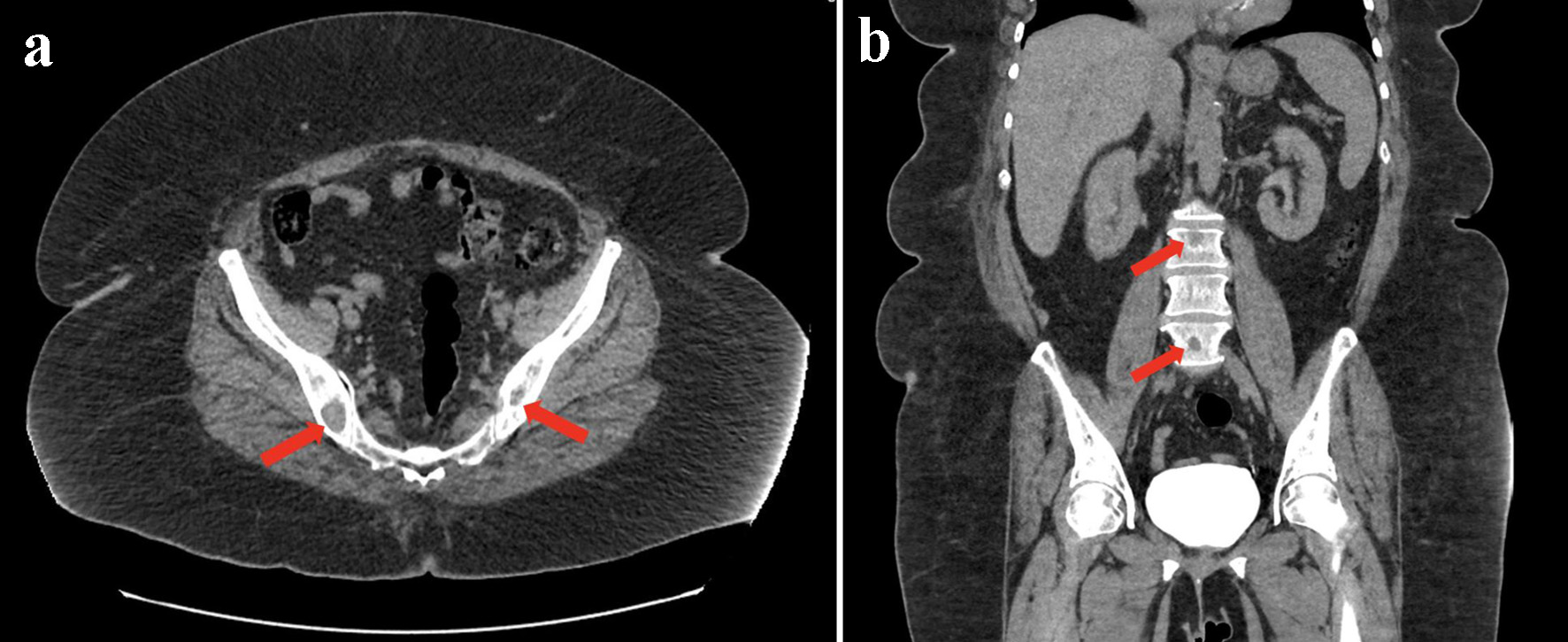 Click for large image | Figure 1. CT of the abdomen and pelvis. (a) Axial view demonstrating prominent osteolytic neoplastic lesions within the pelvis (arrows). (b) Coronal view demonstrating prominent osteolytic neoplastic lesions within the spine (arrows). CT: computed tomography. |
A right iliac bone biopsy was performed with histology demonstrating metastatic carcinoma (Fig. 2a) with epithelial cells staining positive for CAM5.2, CK7 (Fig. 2b), and OSCAR but negative for CK20, E-cadherin, and p63. Additional immunohistochemical staining was consistent with a breast cancer origin, including focal positivity for GATA3 (Fig. 2c), mammaglobin (Fig. 2d), and GCDFP-15 (Fig. 2e). Subsequent mammography and ultrasound identified a 1 × 0.9 × 0.8 cm lesion in the left breast with pathology confirming grade 2 invasive lobular carcinoma (ILC) (Fig. 2f), ER- and PR-positive (> 90%), HER2-negative, with a low mitotic index.
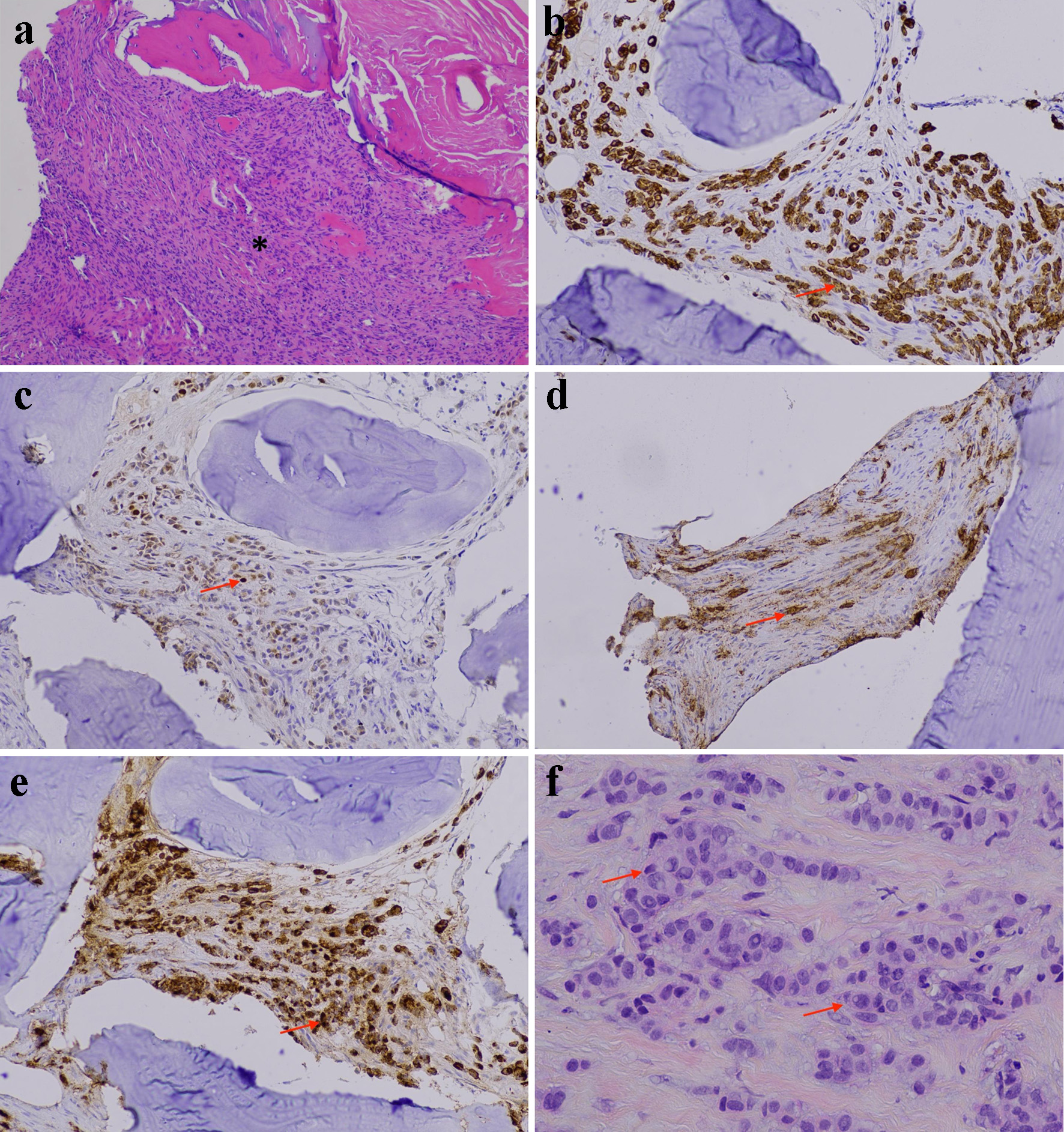 Click for large image | Figure 2. Invasive lobular breast carcinoma. (a) Right iliac bone lesion showing metastatic carcinoma (asterisk) (H&E stain, original magnification, × 100). (b) Strong positive immunostaining for CK7 in the metastatic neoplastic cells (arrow) (immunohistochemistry, original magnification, × 200). (c) Focal positive immunostaining for GATA3 in the metastatic neoplastic cells (arrow) (immunohistochemistry, original magnification, × 200). (d) Focal positive immunostaining for mammaglobin in the metastatic neoplastic cells (arrow) (immunohistochemistry, original magnification, × 200). (e) Strong positive immunostaining for GCDFP-15 in the metastatic neoplastic cells (arrow) (immunohistochemistry, original magnification, × 200). (f) Breast mass biopsy showing invasive lobular carcinoma, grade 2 (arrow) (H&E stain, original magnification, × 400). H&E: hematoxylin and eosin. |
The patient was officially diagnosed with stage IV HR+, HER2-negative ILC with diffuse osseous metastases. Baseline liver function tests (LFTs) were within normal limits. She had no history of liver disease, alcohol use, or recent medication changes. Eastern Cooperative Oncology Group (ECOG) performance status was 1 at presentation.
Treatment course and adverse effects
Ribociclib (600 mg daily for 21 days followed by a 7-day drug holiday) combined with anastrozole (1 mg daily) was initiated approximately 2 weeks after diagnosis. Concurrent medications included insulin, losartan, amlodipine, and sertraline. Approximately 4 weeks into treatment, the patient was hospitalized for acute decompensated heart failure and significant bilateral lower extremity edema. Due to uncertainty of whether this exacerbation was attributed to ribociclib (given a known 12-15% risk of peripheral edema) versus another medication (such as amlodipine or anastrozole), the dose of ribociclib was reduced to 200 mg daily for 21 days, followed by 7 days off.
Approximately 8 weeks into treatment, following two cycles of ribociclib, the patient developed progressive fatigue, jaundice, and dark urine. Laboratory evaluation revealed grade 3 transaminitis (ALT 1,825 U/L, AST 1,536 U/L), alkaline phosphatase (ALP) 278 U/L, total bilirubin 18.1 mg/dL, direct bilirubin 5.1 mg/dL, international normalized ratio (INR) 1.4, and albumin 2.1 g/dL (Fig. 3). Due to significant LFT elevation, ribociclib was immediately discontinued. Despite ribociclib discontinuation, LFTs continued to worsen for several weeks (ALT peaking at 1,967 U/L and AST 1,621 U/L) before stabilizing soon after the patient was started on intravenous NAC (15 g infused over 1 h followed by 5 g infused in the next 4 h, 10 g infused over 16 h, and 625 mg/h for the following 6 days). Over the following 6 weeks post NAC therapy, ALT and AST levels normalized (Fig. 3). Bilirubin, however, continued to rise for several weeks before slowly normalizing (Fig. 3).
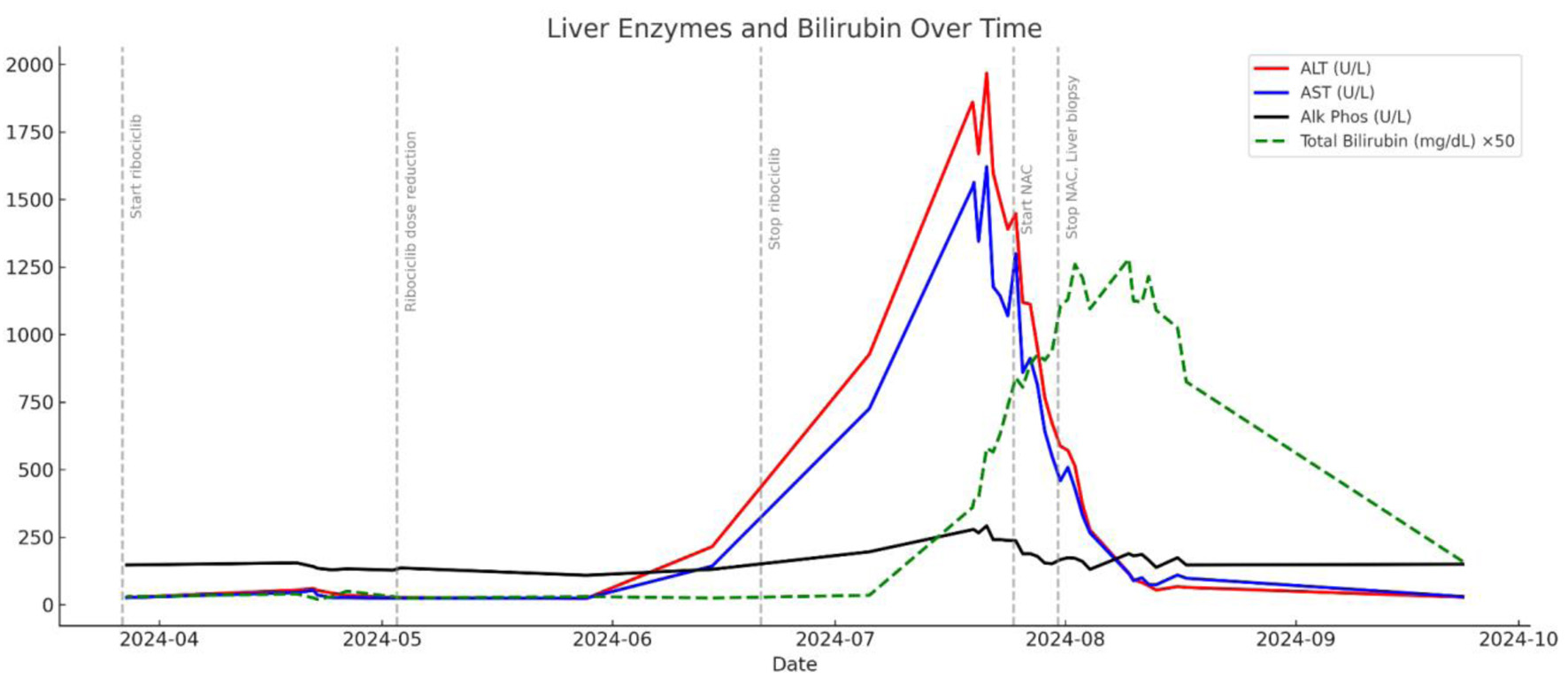 Click for large image | Figure 3. Course of liver enzyme and bilirubin elevation and declination in relation to ribociclib administration, discontinuation, and NAC treatment. NAC: N-acetylcysteine. |
Right upper quadrant ultrasound revealed hepatomegaly and a mildly echogenic liver with some areas of mild surface nodularity, suggestive of steatotic liver disease or fibrofatty cirrhosis. Comprehensive workup ruled out viral hepatitis (hepatitis A, B, C, E, cytomegalovirus (CMV), Epstein-Barr virus (EBV)) and autoimmune hepatitis. Imaging, including MRI and magnetic resonance cholangiopancreatography (MRCP), excluded biliary obstruction.
Liver biopsy
Liver biopsy revealed confluent zonal centrilobular hepatocyte necrosis without significant inflammatory infiltrate, favoring DILI (Fig. 4a-c). The trichrome (Fig. 4d) and reticulin stains (Fig. 4e) demonstrated a collapsed reticulin framework in zone 3 due to confluent hepatocyte necrosis. To provide DILI-specific semiquantitative context, histologic features were summarized using the drug-induced liver injury network (DILIN) histopathology instrument. The overall pattern was hepatocellular with zone 3-predominant confluent necrosis; lobular and portal inflammation were mild; cholestasis and bile-duct injury were not identified; ductular reaction was absent; eosinophils were rare to absent; granulomas and steatosis were not identified; and there was no fibrosis, with reticulin collapse in areas of necrosis.
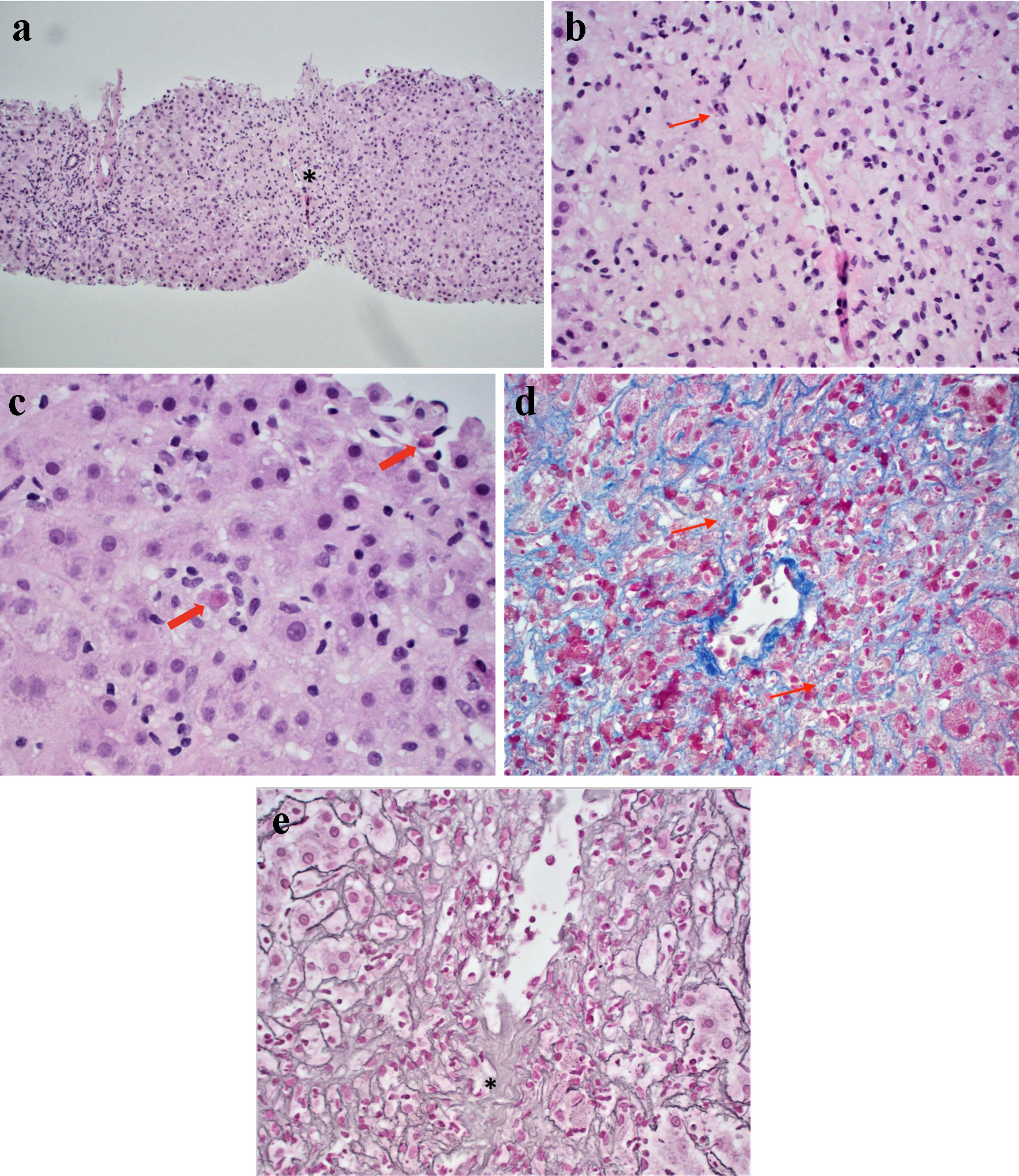 Click for large image | Figure 4. Liver biopsy histology. (a) Centrilobular confluent hepatocyte necrosis (asterisk) with mild lymphocytic portal and lobular inflammation (H&E stain, original magnification, × 100). (b) Higher magnification of extensive centrilobular confluent hepatocyte necrosis (arrow) (H&E stain, original magnification, × 200). (c) Individual necrosis (arrows highlighting apoptotic hepatocytes) (H&E stain, original magnification, × 400). (d) Trichrome special stain demonstrating collapsed reticulin fibers (arrows) due to hepatocyte necrosis, it is negative for fibrosis (special stain, original magnification, × 400). (e) Reticulin special stain demonstrating significant hepatic parenchyma collapse (special stain, original magnification, × 400). H&E: hematoxylin and eosin. |
Causality assessment
Causality assessment tools were employed to support the diagnosis of ribociclib-induced liver injury. An R-factor of 20.73 indicated a hepatocellular pattern of liver injury (R ≥ 5) (Table 1). A RUCAM score of approximately 10 points supported a highly probable association with ribociclib (Table 1). Similarly, a Naranjo algorithm score of 7 indicated a probable adverse drug reaction (Table 1). Together with the histological findings of confluent centrilobular necrosis, these tools strengthen the causal attribution of ribociclib as the source of acute hepatic injury.
 Click to view | Table 1. Causality Assessment Tools Applied to Ribociclib-Induced Liver Injury |
Follow-up
Given the severity of ribociclib-induced DILI, rechallenge with another CDK4/6 inhibitor was deemed unsafe. Instead, the patient’s treatment was continued with anastrozole monotherapy. Follow-up imaging demonstrated stable disease, and no further hepatic complications were reported. Regular monitoring of liver function and imaging is ongoing with no disease progression 13 months following diagnosis.
| Discussion | ▴Top |
In the present case, we report a postmenopausal woman with HR-positive/HER2-negative metastatic ILC who developed severe hepatocellular DILI 8 weeks after starting ribociclib with anastrozole. The case is notable for biopsy-confirmed submassive, zone-3-predominant confluent necrosis - an infrequently reported pattern in ribociclib hepatotoxicity - and for improvement in liver tests after intravenous N-acetylcysteine (NAC) despite initial worsening after drug withdrawal.
Ribociclib, a selective CDK4/6 inhibitor, plays a pivotal role in managing HR-positive, HER2-negative advanced breast cancer by targeting the cyclin D-CDK4/6-Rb axis, halting tumor proliferation through inhibition of the G1-to-S phase cell cycle transition [4, 5]. Ribociclib is generally well-tolerated, with adverse effects such as neutropenia, fatigue, and QT interval prolongation observed in a minority of patients in clinical trials [6, 7]. However, liver injury has emerged as a critical concern due to its comparatively high frequency of grade 3-4 ALT/AST elevations among the CDK4/6 inhibitors. In the MONALEESA-2, MONALEESA-3, and MONALEESA-7 clinical trials, grade 3 or 4 ALT elevations occurred in up to 9.3%, 8.5%, and 5% of patients treated with ribociclib, respectively [1-3]. Whereas in the MONARCH-3 trial (abemacilib) and PALOMA trials (palbociclib), grade 3 or 4 transaminitis occurred in up to 5.8% and 3-4% of patients, respectively [8-10]. Real-world studies have also shown evidence of liver injury similar to that in clinical trials. In a retrospective analysis of 217 patients with metastatic breast cancer treated with CDK4/6 inhibitors, Buller et al reported transaminitis in 9.4% of patients treated with ribociclib with median time to onset of grade > 3 hepatotoxicity being 85 days [11]. Similarly, in a retrospective analysis of 356 patients with HR+/HER2-negative metastatic breast cancer treated with ribociclib plus endocrine therapy, Abdel-Razeq et al reported transaminitis occurrence in 5.9% of patients with 21.4% of all cases of drug cessation attributed to hepatotoxicity [12].
In the current case, ALT/AST values peaked at 1,967/1,621 U/L, and liver biopsy demonstrated significant parenchymal confluent necrosis. At the time of writing, the current case represents one of very few reports showing submassive hepatocyte necrosis secondary to ribociclib. Schaeffer et al documented a similar pattern of injury in a 41-year-old female whose liver biopsy revealed 30% parenchymal necrosis, predominantly in zone III, along with moderate lymphohistiocytic inflammation and mild cholestasis [13]; the patient’s ALT peaked at 2,896 U/L and AST at 875 U/L, with bilirubin reaching 353 µmol/L [13]. Similarly, in a retrospective analysis of 22 cases of DILI secondary to CDK4/6 inhibitors (ribociclib and abemaciclib) by Meunier et al, liver biopsies performed on 12 patients demonstrated centrilobular hepatitis with some cases showing foci of necrosis; however, the quantified extent of necrosis in these cases was not reported [14].
To substantiate attribution of DILI to ribociclib, we applied structured causality tools - R-factor, RUCAM, and Naranjo - in conjunction with histopathologic correlation. The RUCAM score, developed by Danan and Benichou in 1993, is the most widely validated and liver-specific tool for assessing DILI causality [15]. It incorporates time to onset, dechallenge response, exclusion of alternative causes, risk factors, previous hepatotoxicity reports, and results of rechallenge [15]. Updated versions have improved interobserver reproducibility and are now commonly used in both clinical trials and real-world reporting [16]. The R-factor is a biochemical classification index used to categorize the pattern of liver injury (hepatocellular, cholestatic, or mixed) by comparing the relative elevations of ALT and ALP [17]. This classification not only aids in differential diagnosis but also correlates with prognosis, as hepatocellular DILI carries a higher risk of acute liver failure [18]. Originally designed for general adverse drug reaction (ADR) evaluation, the Naranjo algorithm assigns a probability score based on temporal association, dechallenge/rechallenge responses, alternative causes, and prior literature [19]. Though not liver-specific, it provides supportive evidence, particularly in multi-drug scenarios. In our patient, this structured triad of tools strengthened the causal attribution of ribociclib as the offending agent, supported further by histologic evidence of centrilobular necrosis on liver biopsy.
The pathophysiology underlying ribociclib-induced hepatotoxicity is presumed to be multifaceted. One of the proposed mechanisms of ribociclib-induced liver injury is the formation of reactive metabolites during hepatic metabolism, which can cause oxidative stress and hepatocellular damage [20]. The piperazine ring in ribociclib has been identified as a contributing factor to its toxicity, as hydroxylation near nitrogen atoms can generate iminium ions which may covalently bind to hepatocellular macromolecules, disrupting mitochondrial function and inducing hepatocyte apoptosis [20]. The oxidative stress mechanism may explain why some patients, such as in the present case and those presented by Finnsdottir et al, Sozer et al, and Schaeffer et al, show marked improvements in LFTs following NAC supplementation [13, 21, 22]. Additionally, ribociclib has been shown to inhibit the bile salt export pump (BSEP), a key transporter involved in bile acid excretion [23]. This inhibition leads to intracellular bile acid accumulation, triggering hepatocyte injury via oxidative stress, lipid peroxidation, mitochondrial dysfunction, and apoptosis. The BSEP inhibition mechanism may explain the cholestatic features observed in certain cases of ribociclib-induced liver toxicity, such as the present case, in which patients present with jaundice and elevated bilirubin levels [23].
Another factor that exacerbates hepatotoxicity is drug-drug interactions via cytochrome P450 3A4 (CYP3A4) metabolism. Since ribociclib is predominantly metabolized by CYP3A4, co-administration with CYP3A4 inhibitors (such as azole antifungals or macrolides) can significantly increase its plasma concentration, thereby exacerbating hepatotoxicity [24]. Furthermore, interindividual variability in CYP3A4/5 pharmacogenomics can modulate exposure and increase risk for hepatotoxicity; emerging clinical pharmacogenomic studies have identified candidate CYP3A4 variants associated with higher ribociclib exposure, although evidence remains limited and requires validation [25]. Concurrent use of other hepatotoxic drugs, such as statins or acetaminophen, may further enhance the risk of liver injury [6, 7].
Immune-mediated hepatic injury has also been implicated in ribociclib-associated hepatotoxicity. Some cases exhibit autoimmune-like features, such as the presence of antinuclear antibodies (ANA) and anti-mitochondrial antibodies [14]. Liver biopsies in such cases often reveal lymphocytic and eosinophilic infiltration [14]. Additionally, some cases respond to glucocorticoid therapy, further suggesting that an inflammatory immune-driven mechanism may contribute to the observed hepatotoxicity [21].
Effective management of ribociclib-DILI requires early detection, appropriate dose modifications, pharmacological interventions, and a multidisciplinary approach to minimize risks and optimize therapeutic outcomes. Baseline LFTs should be obtained before initiating treatment, with continued monitoring every 2 weeks during the first two cycles, monthly for the next two cycles, and then as clinically indicated [6, 7]. When hepatotoxicity is detected, dose modifications and treatment discontinuation should be implemented based on severity. Mild cases (grade 1 or 2, ALT/AST < 3× upper limit of normal (ULN)) require close monitoring but do not typically necessitate dose modification [6, 7]. Persistent grade 2 elevations lasting more than 7 days (ALT/AST 3 - 5× ULN) warrant temporary treatment interruption, followed by dose reduction upon resolution [6, 7]. Grade 3 elevations (ALT/AST > 5 - 20 × ULN) require immediate dose interruption, with reintroduction at a lower dose (400 mg or 200 mg daily) once liver function normalizes [6, 7]. Severe cases (grade 4, ALT/AST > 20 × ULN or accompanied by jaundice) necessitate permanent discontinuation of ribociclib [14].
Pharmacological interventions can be considered in cases where liver enzyme elevations persist or when severe hepatotoxicity occurs. Corticosteroids, such as prednisone (0.5 - 1 mg/kg daily, tapered over 4 - 8 weeks), are often used for cases exhibiting autoimmune-like features or significant hepatic inflammation [14, 21]. NAC has shown efficacy in managing severe hepatotoxicity, particularly in cases involving hepatic necrosis, with the Prescott regimen (300 mg/kg over 21 h) effectively reducing oxidative stress and promoting hepatic recovery [26]. Additionally, hepatoprotective agents such as ursodeoxycholic acid (UDCA) may be beneficial in cases presenting with cholestatic injury [14].
Minimizing hepatotoxic risk also involves avoiding drug interactions and addressing underlying risk factors. As ribociclib is metabolized by the CYP3A4 enzyme, co-administration with known CYP3A4 inhibitors (e.g., ketoconazole, ritonavir) is particularly problematic, as they can increase drug accumulation and elevate toxicity risk [6, 7]. Additionally, patients should avoid hepatotoxic drugs such as acetaminophen and alcohol [23]. Those with pre-existing liver conditions, including viral hepatitis, non-alcoholic fatty liver disease (NAFLD), or cirrhosis, require closer monitoring due to an increased risk of hepatotoxicity [14].
Additionally, rechallenge and switching to alternative CDK4/6 inhibitors may be considered. If liver enzyme elevations resolve, ribociclib may be reintroduced at a reduced dose (200 mg or 400 mg daily), with close monitoring to prevent recurrence [27]. However, in cases of grade 4 hepatotoxicity, rechallenge is not recommended [21]. Switching to an alternative CDK4/6 inhibitor may be a viable strategy. Palbociclib is associated with a lower risk of hepatotoxicity and may be a safer alternative [27], and abemaciclib, although linked to a higher incidence of gastrointestinal side effects, has demonstrated fewer liver enzyme elevations compared to ribociclib [27].
A multidisciplinary approach, ultimately, is essential for optimizing patient outcomes. Collaboration between oncologists, hepatologists, and pathologists is critical in effectively managing ribociclib-induced hepatotoxicity. Patient education also plays a pivotal role, as individuals should be advised to report symptoms of liver dysfunction, such as jaundice, dark urine, nausea, or fatigue, immediately. Furthermore, patients must be encouraged to adhere to routine LFT monitoring and follow recommended dose modifications to prevent severe hepatotoxicity [7].
Conclusions
Ribociclib-induced hepatotoxicity is a manageable but clinically significant adverse effect in individuals with HR-positive, HER2-negative metastatic breast cancer. Few reports, including the present case, show potential for submassive liver necrosis. Through routine monitoring, timely dose adjustments, pharmacological interventions, and individualized treatment strategies, the risk of liver toxicity can be minimized while maintaining therapeutic efficacy. For patients experiencing hepatotoxicity, treatment decisions should be tailored to the severity of liver enzyme elevations, with options including dose reduction, temporary discontinuation, or transitioning to an alternative CDK4/6 inhibitor. Furthermore, the addition of hepatoprotective agents such as corticosteroid therapy and NAC supplementation may be necessary in cases with persistent worsening of LFTs following ribociclib discontinuation.
Learning points
Ribociclib can cause severe hepatocellular DILI with zone-3 confluent necrosis.
In grade 3-4 hepatocellular injury, permanent discontinuation and non-rechallenge may be prudent.
Intravenous NAC may aid recovery when enzymes worsen after drug withdrawal.
Close LFT monitoring in the first 2 - 3 cycles and review for CYP3A modulators/drug-drug interactions are critical.
Structured causality (R-factor, RUCAM, Naranjo) plus biopsy improves diagnostic certainty and reporting consistency in DILI.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
This manuscript is considered de-identified per the HIPAA Privacy Rule, therefore not requiring the patient’s consent.
Author Contributions
All authors contributed to the final manuscript. CI wrote the main text under the supervision of SG. SG, CI, and IB were involved with final pathological diagnosis, pathologic descriptions, and manuscript revisions.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, Campone M, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29(7):1541-1547.
doi pubmed - Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, Petrakova K, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465-2472.
doi pubmed - Lu YS, Im SA, Colleoni M, Franke F, Bardia A, Cardoso F, Harbeck N, et al. Updated overall survival of ribociclib plus endocrine therapy versus endocrine therapy alone in pre- and perimenopausal patients with HR+/HER2- advanced breast cancer in MONALEESA-7: a phase III randomized clinical trial. Clin Cancer Res. 2022;28(5):851-859.
doi pubmed - Hamilton E, Infante JR. Targeting CDK4/6 in patients with cancer. Cancer Treat Rev. 2016;45:129-138.
doi pubmed - O'Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13(7):417-430.
doi pubmed - Cazzaniga ME, Danesi R, Girmenia C, Invernizzi P, Elvevi A, Uguccioni M, Amaducci L, et al. Management of toxicities associated with targeted therapies for HR-positive metastatic breast cancer: a multidisciplinary approach is the key to success. Breast Cancer Res Treat. 2019;176(3):483-494.
doi pubmed - Cazzaniga ME, Ciaccio A, Danesi R, Duhoux FP, Girmenia C, Zaman K, Lindman H, et al. Late onset toxicities associated with the use of CDK 4/6 inhibitors in hormone receptor positive (HR+), human epidermal growth factor receptor-2 negative (HER2-) metastatic breast cancer patients: a multidisciplinary, pan-EU position paper regarding their optimal management. The GIOCONDA project. Front Oncol. 2023;13:1247270.
doi pubmed - Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, Park IH, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638-3646.
doi pubmed - Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, Harbeck N, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016;375(20):1925-1936.
doi pubmed - Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, Colleoni M, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379(20):1926-1936.
doi pubmed - Buller W, Pallan L, Chu T, Khoja L. CDK4/6 inhibitors in metastatic breast cancer, a comparison of toxicity and efficacy across agents in a real-world dataset. J Oncol Pharm Pract. 2023;29(8):1825-1835.
doi pubmed - Abdel-Razeq H, Sharaf B, Khater S, Baidoun HJ, Bani Hani H, Taqash A, El Khatib O, et al. Clinical outcomes of patients treated with ribociclib in combination with aromatase inhibitors or fulvestrant for HR-positive, HER2-negative metastatic breast cancer, real-world data from a low-resourced country. Immunotargets Ther. 2024;13:501-512.
doi pubmed - Schaeffer S, Lutz C, Dobbie M, Terracciano LM, Matter M, Vosbeck J, Heim MH, et al. Ribociclib-induced liver injury: a case report. Front Oncol. 2023;13:1256783.
doi pubmed - Meunier L, De Martin E, Delire B, Jacot W, Guiu S, Zahhaf A, Larrey D, et al. CDK4/6 inhibitor-induced liver injury: Clinical phenotypes and role of corticosteroid treatment. JHEP Rep. 2024;6(7):101098.
doi pubmed - Danan G, Benichou C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46(11):1323-1330.
doi pubmed - Danan G, Teschke R. RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci. 2015;17(1).
doi pubmed - Kleiner DE, Chalasani NP, Lee WM, Fontana RJ, Bonkovsky HL, Watkins PB, Hayashi PH, et al. Hepatic histological findings in suspected drug-induced liver injury: systematic evaluation and clinical associations. Hepatology. 2014;59(2):661-670.
doi pubmed - Andrade RJ, Lucena MI, Fernandez MC, Pelaez G, Pachkoria K, Garcia-Ruiz E, Garcia-Munoz B, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129(2):512-521.
doi pubmed - Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
doi pubmed - Alsubi TA, Attwa MW, Bakheit AH, Darwish HW, Abuelizz HA, Kadi AA. Correction: In silico and in vitro metabolism of ribociclib: a mass spectrometric approach to bioactivation pathway elucidation and metabolite profiling. RSC Adv. 2020;10(40):23930.
doi pubmed - Finnsdottir S, Sverrisdottir A, Bjornsson ES. Hepatotoxicity associated with ribociclib among breast cancer patients. Acta Oncol. 2021;60(2):195-198.
doi pubmed - Sozer Karadagli S, Gursoy P. Liver toxicity with ribociclib in a patient with metastatic hormone receptor positive postmenopausal breast cancer. J Oncol Pharm Pract. 2024;30(2):404-407.
doi pubmed - She Y, Guo Z, Zhai Q, Liu J, Du Q, Zhang Z. CDK4/6 inhibitors in drug-induced liver injury: a pharmacovigilance study of the FAERS database and analysis of the drug-gene interaction network. Front Pharmacol. 2024;15:1378090.
doi pubmed - Braal CL, Jongbloed EM, Wilting SM, Mathijssen RHJ, Koolen SLW, Jager A. Inhibiting CDK4/6 in Breast Cancer with Palbociclib, Ribociclib, and Abemaciclib: Similarities and Differences. Drugs. 2021;81(3):317-331.
doi pubmed - Schlam I, Smith DM, Peer C, Sissung T, Schmidt KT, Tan M, Chitalia A, et al. Pharmacokinetics and pharmacogenomics of ribociclib in black patients with metastatic breast cancer the LEANORA study. NPJ Breast Cancer. 2024;10(1):84.
doi pubmed - Hyppolite JJ, Hilzenrat N. Palbociclib-induced severe hepatitis: A case study and literature review. Can Liver J. 2021;4(4):433-437.
doi pubmed - Farhat F, Tarabaih M, Kanj A, Aoun M, Kattan J, Assi T, Awada A. Palbociclib safety and efficacy beyond Ribociclib-induced liver toxicity in metastatic hormone-receptors positive breast cancer patient. Anticancer Drugs. 2020;31(1):85-89.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.