| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com |
Case Report
Volume 16, Number 9, September 2025, pages 366-371
Persistent Impairment of Coronary Microvascular Dysfunction After Percutaneous Coronary Intervention in an Ice Swimming Champion
Gueladio Konea, Matthieu Godina, Alexandre Fuzeaua, Arnaud Verdoncka, Francois Raouxb, c, Jean-Nicolas Dacherd, Laetitia Neuvillerse, Julien Le Moalf, Quentin Landolffa, g
aDepartment of Cardiology, Clinique Saint-Hilaire, Rouen, France
bDepartment of Cardiology, INSEP (Institut National du Sport), Paris, France
cDepartment of Cardiology, Institut Mutualiste Montsouris, Paris, France
dDepartment of Radiology, CHU (Centre Hospitalo-Universitaire) de Rouen, Rouen, France
eDepartment of Cardiology and Radiology, Hopital Prive Clairval, Marseille, France
fDepartment of Radiology, Clinique Saint Hilaire, Rouen, France
gCorresponding Author: Quentin Landolff, Department of Cardiology, Clinique Saint-Hilaire, 76000 Rouen, France
Manuscript submitted June 3, 2025, accepted August 27, 2025, published online September 17, 2025
Short title: CMD After PCI in an Ice Swimming Champion
doi: https://doi.org/10.14740/jmc5150
| Abstract | ▴Top |
Coronary microvascular dysfunction (CMD) is a recognized cause of persistent angina post-percutaneous coronary intervention (PCI), especially in patients without epicardial coronary stenosis. We report a case of a 58-year-old top-level sportsman and world champion ice swimmer with persistent dyspnea despite successful PCI for a mid-left anterior descending artery lesion. Follow-up angiography with optical coherence tomography showed no in-stent restenosis with good stent apposition. Angiography-derived microcirculatory resistance (AMR, Pulse Medical) and cardiac magnetic resonance imaging revealed CMD as the underlying etiology. This case demonstrates the utility and feasibility of AMR in identifying CMD post-PCI and supports its use in the diagnostic workup.
Keywords: Coronary microvascular dysfunction; Percutaneous coronary intervention
| Introduction | ▴Top |
Coronary microvascular dysfunction (CMD) refers to impaired vasodilatory function of the coronary microcirculation in the absence of significant epicardial coronary artery disease. In the setting of post-percutaneous coronary intervention (PCI), coronary microvascular obstruction (MVO) can mainly be caused by distal thromboembolism, circulating blood cells plugging and in situ microvascular thrombosis. MVO is regarded to be the leading pathology behind post-PCI myocardial mal perfusion. It is a frequent finding in 20% to 30% of patients undergoing coronary angiography for suspected coronary artery disease. For example, 3 to 4 million Americans suffer from angina related to coronary microcirculation anomalies [1, 2]. Extreme sports such as swimming in extremely cold water (0 °C) can cause cardiac abnormalities, in particular CMD and cardiac arrhythmias [3]. This case is of particular interest due to the integration of multiple advanced imaging and diagnostic tools, including optical coherence tomography (OCT), fractional flow reserve (FFR), radial wall strain (RWS), angiography-derived microcirculatory resistance (AMR), and cardiac magnetic resonance imaging (MRI). Concerning the RWS (Pulse Medical), it evaluates the biomechanical stress on coronary plaques and may help identify vulnerable lesions; however, the data published to date are still limited [4-6].
| Case Report | ▴Top |
Investigations
A 58-year-old top-level sportsman and world champion ice swimmer presented with non-ST-elevation myocardial infarction (NSTEMI). The patient usually swims for 2 to 20 min in ice-cold water (less than 5 °C) during the winter months from November to March, three times a week, covering a total distance of 40 km per season. His symptoms included limited functional capacity and mild troponin I ultrasensitive (us) elevation: 200 - 300 pg/mL (standard < 14 pg/mL) in the context of transient atrial fibrillation, consistent with a type 2 myocardial infarction (MI). He had no significant past medical history, with the exception of cardiac arrhythmia due to paroxysmal atrial fibrillation. His only cardiovascular risk factor is a hypercholesterolemia.
Diagnosis
Coronary angiography revealed a mid-left anterior descending (LAD) stenosis with a positive FFR (Boston Scientific) to 0.75 (standard > 0.80). OCT (Abbott) confirmed the fibro-lipid plaque stenosis with minimum lumen area (MLA) to 2.3 mm2 (standard > 4 mm2). Subsequent analysis using RWS (Pulse Medical) software determined that the mid-LAD stenosis was vulnerable with a high risk of MI with max RWS = 17% (standard < 14%) (Figs. 1 and 2). The echocardiography was normal with a left ventricular ejection fraction (LVEF) at 55%.
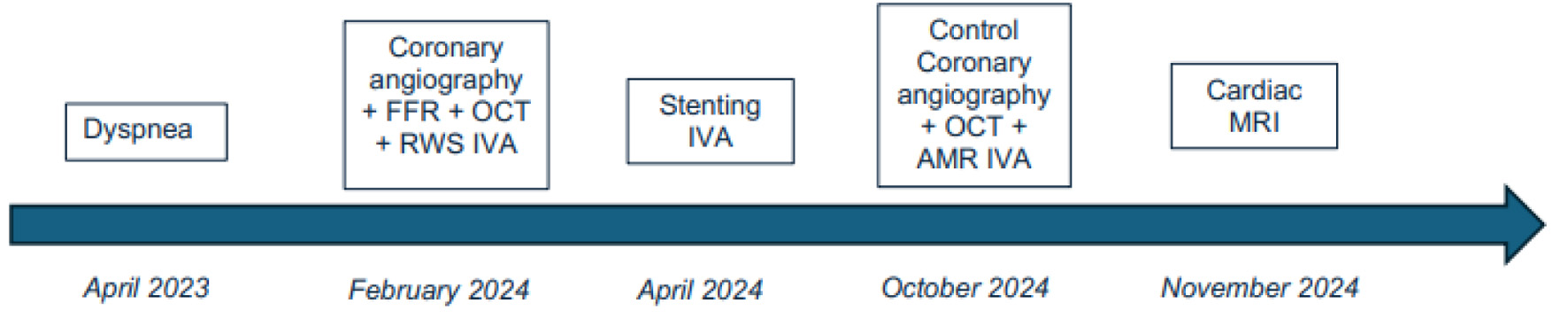 Click for large image | Figure 1. Timeline figure summarizing symptoms, diagnostics, interventions, and follow-up. |
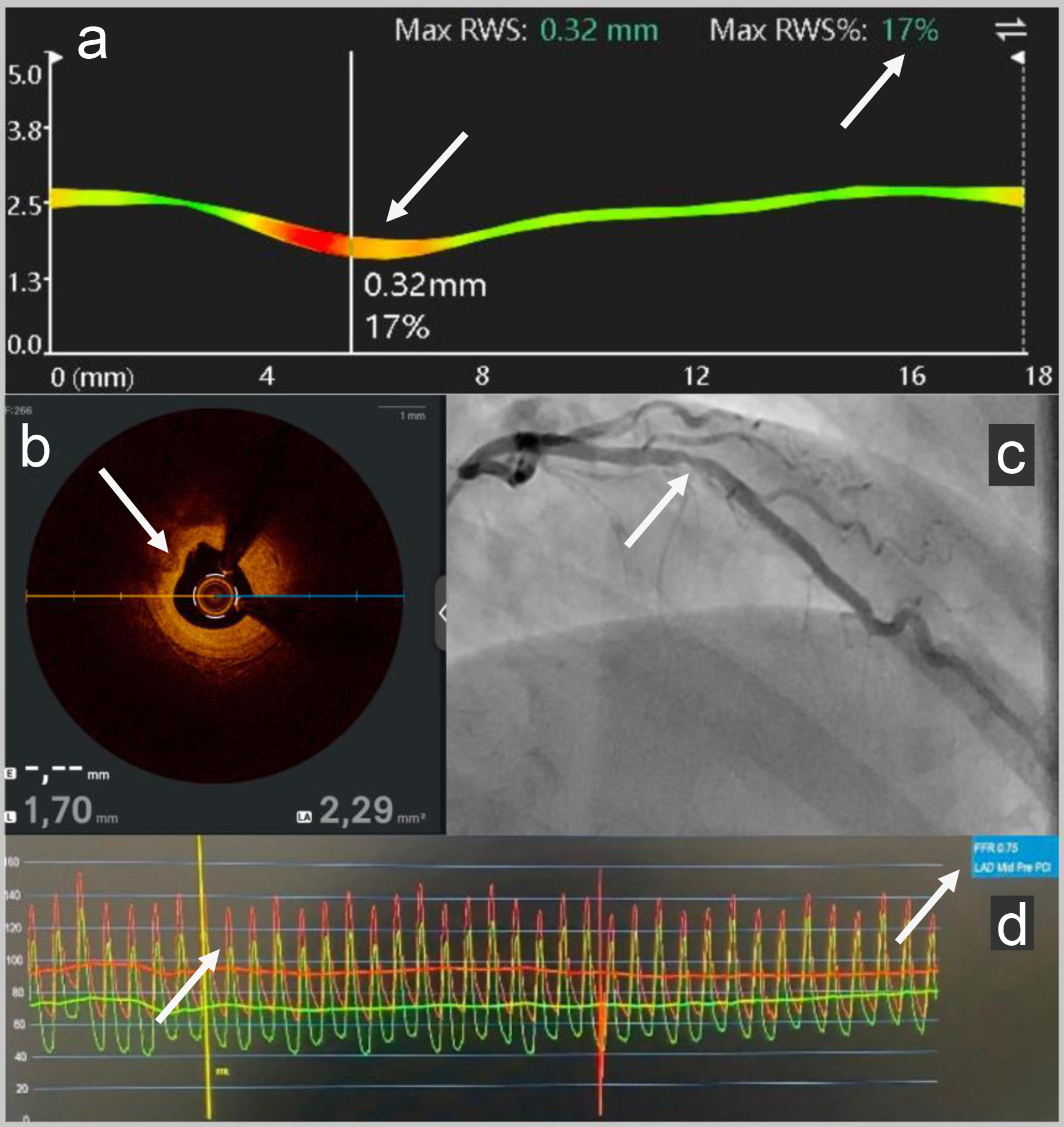 Click for large image | Figure 2. (a) RWS pre-PCI: Subsequent analysis using RWS (Pulse Medical) software determined the vulnerability of the mid-LAD stenosis with a high risk of myocardial infarction with pathological max RWS = 17%. (b) OCT pre-PCI: Intravascular coronary imaging: OCT (Abbott) showed a fibro-lipid plaque (the intimal layer primarily appears bright and relatively homogeneous; it causes hardly any shadowing because of the fibrous tissue) in the mid-LAD with MLA to 2.3 mm2 (standard > 4 mm2). (c) Angiography pre-PCI: Coronary angiography revealed a mid-LAD coronary artery stenosis. (d) FFR pre-PCI: The LAD FFR (Boston Scientific) was positive to 0.75 (standard > 0.80). RWS: radial wall strain; PCI: percutaneous coronary intervention; LAD: left anterior descending; OCT: optical coherence tomography; MLA: minimum lumen area; FFR: fractional flow reserve. |
Treatment
He underwent a PCI of the LAD with a bioresorbable magnesium drug-eluting stent Freesolve (Biotronik), with the aim of preserving long-term endothelial function and without leaving any scaffold in his coronary arteries (Figs. 1 and 3).
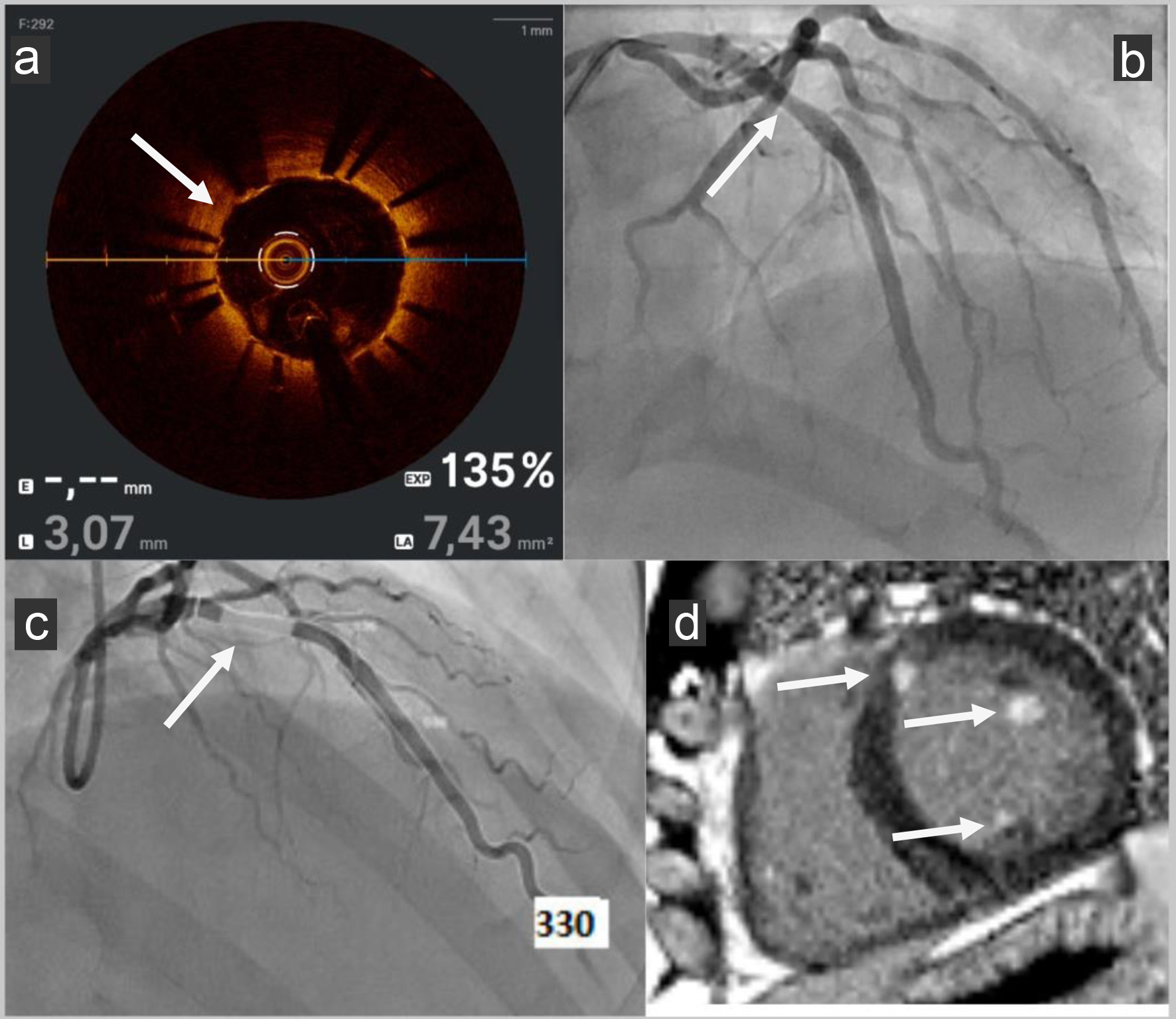 Click for large image | Figure 3. (a) OCT post-PCI: OCT (Abbott) showed good stent apposition (no space greater: malapposition than 0.4 mm2 between the stent struts and the intimal wall of the artery) with no ISR with MLA to 7.4 mm2 (standard > 4 mm2). (b) Angiography post-PCI: LAD PCI was performed with a bioresorbable drug-eluting stent Freesolve (Biotronik). (c) AMR post-PCI: AMR (Pulse Medical) was calculated from one coronary angiography view, yielding a pathological value of 330 mm Hg s/m (standard < 250 mm Hg s/m). (d) Cardiac MRI post-PCI: Late enhancement sequences in cardiac MRI were pathological with presence of basal and antero-septal focal intra- and sub-endocardial enhancement, involvement of two mitral pillars and a right intra ventricular mitral cord. OCT: optical coherence tomography; PCI: percutaneous coronary intervention; ISR: in-stent restenosis; MLA: minimum lumen area; LAD: left anterior descending; AMR: angiography-derived microcirculatory resistance; MRI: magnetic resonance imaging. |
Follow-up and outcomes
Despite successful revascularization, the patient continued to experience limited exercise capacity during his usual swimming activities. He had no chest pain or dyspnea or asthenia but felt limited in his efforts while continuing to do his usual physical activity. Follow-up angiography was performed with OCT (Abbott), showing good stent apposition (no malapposition > 0.4 mm2) with no in-stent restenosis (ISR) (standard > 4 mm2) (Figs. 1 and 3). His troponin I us levels, on average between 200 and 300 pg/mL (standard < 14 pg/mL), reached a peak of 6,025 pg/mL following physical exertion, the patient remaining pain-free.
We decided to assess coronary microvascular resistance by virtual AMR by Pulse Medical software during remote control coronary angiography of NSTEMI. AMR (Pulse Medical) was calculated from one coronary angiography view, yielding a value of 330 mm Hg s/m, indicating significant microvascular dysfunction (standard < 250 mm Hg s/m) (Figs. 1 and 3) [7].
Magnetic resonance flow reserve (MRFR) by cardiac MRI assessed myocardial perfusion was not performed because of the lack of software in our center. MRI identified subnormal LVEF (53%) and late gadolinium enhancement involving the basal and antero-septal regions, consistent with prior ischemic injury (presence of basal and antero-septal focal intra- and sub endocardial enhancement, involvement of two mitral pillars and a right intra ventricular mitral cord). It also revealed no left ventricular hypertrophy or increased extracellular volume. There was bi-atrial dilatation: left atrium 24 cm2 and right atrium 29 cm2. T1 relaxometry was rather low in relation to sporting activity, with a mean value of 967 ms (standard 950 - 1,000 ms), with a higher value in the antero-septal basal area (late enhancement zone). T2 and T2* relaxometry were normal, with no evidence of acute edema (Figs. 1 and 3).
| Discussion | ▴Top |
This case highlights the value of AMR (Pulse Medical) in assessing CMD post-PCI. Persistent symptoms on exertion without chest pain following PCI and without residual stenosis can be caused by CMD, which is characterized by elevated coronary microvascular resistance in the absence of significant epicardial coronary artery disease.
Several techniques are available to assess microvascular resistance [7]. However, index of microcirculatory resistance (IMR, Abbott) is limited by the use of hyperemic agents and pressure wires, leading to longer procedure times and increased costs, but would be useful in this case with a patient with NSTEMI [8, 9]. In our patient’s case, we decided not to re-evaluate the patient for a new coronary angiography with analysis of the coronary microvascular resistance by IMR (Abbott). Indeed, the AMR study (Pulse Medical) enabled us to carry out a retrospective analysis of the coronary microvascular resistance without reconvening the patient and taking even the moderate risk of a new invasive procedure. Similarly, cardiac magnetic resonance (CMR) is the gold standard for myocardial perfusion imaging and tissue viability assessment [10]. However, it faces logistical challenges, including limited availability, prolonged examination times, and high costs. We could also have carried out cold water tests, or even a methergin test, but these were not performed as they were invasive. Although invasive IMR is the gold standard, technical (i.e., the side effects of vasodilator agents required for stable hyperemia induction and complications related to vessel wiring) and economic issues hamper its use in routine clinical practice. To address these limitations, AMR (Pulse Medical) has emerged as a good alternative. AMR (Pulse Medical) is a virtual software, pressure-wire-free, adenosine-free and angiography-derived metric that simplifies the assessment of coronary microvascular resistance, offering a more accessible and cost-effective solution. Few studies showed a good correlation between AMR and IMR [11-14]. AMR utilized coronary angiography images, automatically selecting frames displaying good contrast fill and fully exposed lumen contours as analysis frames. It also automatically delineates the lumen boundaries of the target vessel and its major side branches. The reference vessel diameter was reconstructed using Murray’s fractal law for bifurcation, which considers the buckling phenomenon occurring at the bifurcation. In the diagnostic population, target vessels were assessed for measurements of IMR using AMR and using: AMR = Pd/Velocity hpy = Pa × µQFR/Velocity hpy (standard < 250 mm Hg s/m)
IMR is the established standard to evaluate the presence of CMD. Physiological parameters were quantified utilizing a guidewire equipped with pressure and temperature sensors. The arterial pressure (Pa) upstream of the lesion and the distal arterial pressure (Pd) were recorded. FFR during engorgement was calculated as the mean ratio of Pd/Pa. Patients participating in the diagnostic population study underwent IMR measurement. A thermodilution curve was generated by injecting 3 mL of room-temperature saline into the coronary artery, and the mean time to engorgement (TmnH) was calculated on three consecutive occasions. The impact of collateral circulation on IMR was adjusted considering the coronary wedge pressure (Pw). IMR was quantified using the formula: IMR = (Pa × TmnH × [Pd - Pw])/(Pa - Pw) (standard < 25)
Fan et al found a correlation between AMR and IMR (R2 = 0.807; P < 0.01), with no statistically significant difference in this correlation observed between smokers and non-smokers. Furthermore, a clear positive correlation was maintained in both smokers (R2 = 0.803, P < 0.01) and non-smokers (R2 = 0.907, P < 0.01). The diagnostic performance of AMR was found to be high, with an area under the curve (AUC) of 0.90 and a diagnostic accuracy of 80.6% [11, 12].
In our case, both AMR (Pulse Medical) and CMR were consistent with CMD as the cause of the patient’s symptoms. While AMR (Pulse Medical) quantified the hemodynamic burden with increased risk of major adverse cardiovascular events (MACEs) when superior to 250 mm Hg s/m, CMR provided detailed information such as ischemic area and excluded MI or fibrosis as alternative causes [10, 15]. The combination of AMR (Pulse Medical) and CMR facilitated the initiation of targeted vasodilator therapy, including calcium channel blockers with moderate symptoms improvement after 3 months in this case. The integration of these diagnostic tools highlights the importance of a multimodal approach to evaluate post-PCI angina and optimize therapeutic strategies. Further research is needed to standardize diagnostic protocols, establish universal cut-off values for CMD assessment, and improve the management of CMD in clinical practice.
Troponin elevation after ultra sports is well described in ultra marathons [16-19]. There are no published studies on troponin elevation after swimming in cold or extremely cold water, only after a triathlon (swimming, cycling, and running) [20]. High troponin level has been described after scuba diving, which does not correspond to our case, since hypoxia and depth are also factors in scuba diving [21-23]. CMD is sometimes found in athletes. The patient has never had exercise stress. Van de Sande et al showed that athletes with abnormal exercise stress and myocardial perfusion scintigraphy results indicative for myocardial ischemia and without obstructive coronary artery disease showed a lower coronary flow reserve when compared with non-athletes with a low-to-intermediate a priori risk of coronary artery disease, suggesting an attenuated coronary microvascular function. Higher endothelin-1 concentrations in athletes suggest that endothelial-dependent dysfunction may be an important determinant of the attenuated coronary microvascular function [24].
The association between CMD and exposure to cold is well known. Blood pressure rises as a result of peripheral vasoconstriction, sympathetic activation, and increased muscular tone brought on by cold exposure. Furthermore, exposure to cold promotes cholesterol crystallization in atherosclerotic plaques. Demand ischemia and/or atherosclerotic plaque rupture can result from the interaction of sympathetic activity, high blood pressure, and cholesterol crystallization [25]. Extreme cold within the preceding 48 h has been associated with a significantly increased risk of MI (relative risk (RR) of 1.36) [26]. A temperature decrease of 1 °C below the 24 °C threshold was correlated with a 3.7% increase in MI-related hospitalizations [27]. A study revealed an increased risk of MI at temperatures below 12 °C (hazard ratio (HR): 0.988) in Shanghai, China [28]. There is a risk of acute cardiovascular disease mortality in Swiss individuals during cold temperatures (odds ratio of 1.15), specifically at the fifth percentile of the lowest daily mean temperature (-3 °C) versus the optimal temperature (20 °C). A previous study compared the RRs of ischemic heart disease mortality due to extreme cold. The extreme cold (first percentile) verse minimum mortality temperature was estimated at RR of 1.3 [29]. The study of Chen et al corroborates the association between cold weather and death from MI [30].
Conclusion
Multiple diagnostic tools: IMR, AMR, MRI can help the clinician to assess a CMD post-PCI. Regular prolonged swimming in extremely cold water (0 °C) could probably lead to CMD.
Learning points
FFR, RWS, and OCT are essential tools for assessing the severity of coronary artery lesions. PCI with a bioresorbable drug-eluting stent avoids endothelial dysfunction, neo-atherosclerosis progression, late thrombosis events, and stent fractures after device resorption by hydrolysis. This can be useful, particularly for top-level athletes. IMR, AMR, and cardiac MRI are essential tools for assessing CMD. Regular prolonged swimming in extremely cold water (0 °C) could probably lead to CMD with high troponin elevation.
Acknowledgments
We would like to thank Naomi Stone for her English corrections.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
The patient’s signed consent has been obtained.
Author Contributions
MG and QL performed the procedure. GK and QL wrote the document. MG, AF, FR, and AV reviewed the document. JND performed cardiac MRI. JND, LN, and JLM analyzed cardiac MRI.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
| References | ▴Top |
- Sezer M, van Royen N, Umman B, Bugra Z, Bulluck H, Hausenloy DJ, Umman S. Coronary microvascular injury in reperfused acute myocardial infarction: a view from an integrative perspective. J Am Heart Assoc. 2018;7(21):e009949.
doi pubmed - Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, et al. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J Am Coll Cardiol. 2018;72(23 Pt A):2841-2855.
doi pubmed - Fuzeau A. Book "Ice fight". Chapter 31 Cordial feelings P178-9. Chapter 38 Crazy and cold P218-221. The Book Edition. 2024.
- Gallen C, Boussofara A, Landolff Q. How to avoid a myocardial infarction with radial wall strain? J Invasive Cardiol. 2025;37(6).
doi pubmed - Skalidis I, D'Angelo L, Garot J, Amabile N. Radial wall strain in the assessment of non-flow-limiting unstable plaques in MINOCA. JACC Cardiovasc Interv. 2025;18(9):1190-1191.
doi pubmed - Wang ZQ, Xu B, Li CM, Guan CD, Chang Y, Xie LH, Zhang S, et al. Angiography-derived radial wall strain predicts coronary lesion progression in non-culprit intermediate stenosis. J Geriatr Cardiol. 2022;19(12):937-948.
doi pubmed - Fearon WF, Kobayashi Y. Cut-off values for invasive microvascular dysfunction assessments. Circulation. 2017;136(6):520-533.
- Zhang Y, Pu J, Niu T, Fang J, Chen D, Yidilisi A, Zheng Y, et al. Prognostic value of coronary angiography-derived index of microcirculatory resistance in non-ST-segment elevation myocardial infarction patients. JACC Cardiovasc Interv. 2024;17(16):1874-1886.
doi pubmed - Scarsini R, Shanmuganathan M, Kotronias RA, Terentes-Printzios D, Borlotti A, Langrish JP, Lucking AJ, et al. Angiography-derived index of microcirculatory resistance (IMR(angio)) as a novel pressure-wire-free tool to assess coronary microvascular dysfunction in acute coronary syndromes and stable coronary artery disease. Int J Cardiovasc Imaging. 2021;37(6):1801-1813.
doi pubmed - Takafuji M, Ishida M. Understanding myocardial ischemia: Cardiac magnetic resonance insight into coronary macro- and microcirculation pathophysiology. Current Cardiovascular Imaging Reports. 2024;18:4.
- Fan Y, Fezzi S, Sun P, Ding N, Li X, Hu X, Wang S, et al. In vivo validation of a novel computational approach to assess microcirculatory resistance based on a single angiographic view. J Pers Med. 2022;12(11):1798.
doi pubmed - Qiu Z, Wang Y, Liu Y, Zhou Z, Wang Z. Diagnostic value of angiography-derived index of microcirculatory resistance (AMR) for coronary microcirculatory dysfunction (CMD) and its prognostic significance in patients with chronic coronary syndromes in the smoking population. Medicine (Baltimore). 2024;103(6):e37022.
doi pubmed - Zhang Z, Dai Y, Xue P, Bao X, Bai X, Qiao S, Gao Y, et al. Prediction of microvascular obstruction from angio-based microvascular resistance and available clinical data in percutaneous coronary intervention: an explainable machine learning model. Sci Rep. 2025;15(1):3045.
doi pubmed - Zhang Z, Dai Q, Zhang X, Qiao S, Bao X, Wang K, Xue P, et al. Microcirculatory resistance based on a single angiographic view in ST-segment elevation myocardial infarction patients. BMC Cardiovasc Disord. 2025;25(1):357.
doi pubmed - Kotecha T, Martinez-Naharro A, Boldrini M, Knight D, Hawkins P, Kalra S, Patel D, et al. Automated pixel-wise quantitative myocardial perfusion mapping by CMR to detect obstructive coronary artery disease and coronary microvascular dysfunction: validation against invasive coronary physiology. JACC Cardiovasc Imaging. 2019;12(10):1958-1969.
doi pubmed - Tsai IH, Kao WF, How CK, Li LH, Lin YK, Kung LC, Chiu YH, et al. Cardiac autonomic regulation following a 246-km mountain ultra-marathon: An observational study. Medicine (Baltimore). 2024;103(27):e38756.
doi pubmed - Rubio-Arias JA, Andreu L, Martinez-Aranda LM, Martinez-Rodriguez A, Manonelles P, Ramos-Campo DJ. Effects of medium- and long-distance running on cardiac damage markers in amateur runners: a systematic review, meta-analysis, and metaregression. J Sport Health Sci. 2021;10(2):192-200.
doi pubmed - Liu CH, Li LH, Chang ML, Kao WF, How CK, Lai JI, Lin YK, et al. Electrical cardiometry and cardiac biomarkers in 24-h and 48-h ultramarathoners. Int J Sports Med. 2021;42(11):1035-1042.
doi pubmed - Zebrowska A, Waskiewicz Z, Nikolaidis PT, Mikolajczyk R, Kawecki D, Rosemann T, Knechtle B. Acute responses of novel cardiac biomarkers to a 24-h ultra-marathon. J Clin Med. 2019;8(1):57.
doi pubmed - Legaz-Arrese A, Lopez-Laval I, George K, Jose Puente-Lanzarote J, Castellar-Otin C, Reverter-Masia J, Munguia-Izquierdo D. Individual variability of high-sensitivity cardiac troponin levels after aerobic exercise is not mediated by exercise mode. Biomarkers. 2015;20(4):219-224.
doi pubmed - Zarak M, Perovic A, Dobrovic I, Goreta SS, Dumic J. Galectin-3 and cardiovascular biomarkers reflect adaptation response to scuba diving. Int J Sports Med. 2020;41(5):285-291.
doi pubmed - Marlinge M, Coulange M, Fitzpatrick RC, Delacroix R, Gabarre A, Laine N, Cautela J, et al. Physiological stress markers during breath-hold diving and SCUBA diving. Physiol Rep. 2019;7(6):e14033.
doi pubmed - Eichhorn L, Doerner J, Luetkens JA, Lunkenheimer JM, Dolscheid-Pommerich RC, Erdfelder F, Fimmers R, et al. Cardiovascular magnetic resonance assessment of acute cardiovascular effects of voluntary apnoea in elite divers. J Cardiovasc Magn Reson. 2018;20(1):40.
doi pubmed - van de Sande D, Barneveld PC, Hoogsteen J, Doevendans PA, Kemps HMC. Coronary microvascular function in athletes with abnormal exercise test results. Neth Heart J. 2019;27(12):621-628.
doi pubmed - Khraishah H, Alahmad B, Ostergard RL, Jr., AlAshqar A, Albaghdadi M, Vellanki N, Chowdhury MM, et al. Climate change and cardiovascular disease: implications for global health. Nat Rev Cardiol. 2022;19(12):798-812.
doi pubmed - Madrigano J, Mittleman MA, Baccarelli A, Goldberg R, Melly S, von Klot S, Schwartz J. Temperature, myocardial infarction, and mortality: effect modification by individual- and area-level characteristics. Epidemiology. 2013;24(3):439-446.
doi pubmed - Goggins WB, Chan EY, Yang CY. Weather, pollution, and acute myocardial infarction in Hong Kong and Taiwan. Int J Cardiol. 2013;168(1):243-249.
doi pubmed - Huang X, Ma W, Law C, Luo J, Zhao N. Importance of applying Mixed Generalized Additive Model (MGAM) as a method for assessing the environmental health impacts: Ambient temperature and Acute Myocardial Infarction (AMI), among elderly in Shanghai, China. PLoS One. 2021;16(8):e0255767.
doi pubmed - Alahmad B, Khraishah H, Roye D, Vicedo-Cabrera AM, Guo Y, Papatheodorou SI, Achilleos S, et al. Associations between extreme temperatures and cardiovascular cause-specific mortality: results from 27 countries. Circulation. 2023;147(1):35-46.
doi pubmed - Chen YH, Liu IH, Hsiao CC, Cheng CG, Cheng CA. Effects of exposure to air pollution and cold weather on acute myocardial infarction mortality. Atmosphere. 2025;16(4):469.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.