| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com |
Case Report
Volume 000, Number 000, July 2025, pages 000-000
A Heart of Myeloid: Extramedullary Chronic Myelomonocytic Leukemia-2 Presenting as a Myeloid Sarcoma of the Pericardium Causing Recurrent Pericardial Effusions
Austin Frischa, f, Rohan Boyapatib, Ruja Parikhb, Geetha Menezesc, Niharika Tipirnenid, Germame Ajeboe, Danielle Shafere
aDepartment of Internal Medicine, Inova Fairfax Medical Center, Falls Church, VA, USA
bUniversity of Virginia School of Medicine, Charlottesville, VA, USA
cDepartment of Pathology, Inova Fairfax Medical Center, Falls Church, VA, USA
dDepartment of Medical Critical Care Services, Inova Fairfax Medical Center, Falls Church, VA, USA
eInova Schar Cancer Center, Inova Fairfax Medical Center, Falls Church, VA, USA
fCorresponding Author: Austin Frisch, Department of Internal Medicine, Inova Fairfax Hospital, Fairfax, VA, USA
Manuscript submitted May 22, 2025, accepted June 23, 2025, published online July 8, 2025
Short title: CMML-2 Presenting as a Myeloid Sarcoma
doi: https://doi.org/10.14740/jmc5144
| Abstract | ▴Top |
Chronic myelomonocytic leukemia (CMML) is a rare neoplasm that has a roughly 15-30% chance of transforming into acute myeloid leukemia (AML). Acute leukemias have been known to cause pleural or pericardial effusions but having CMML transform into AML while presenting as a cardiac myeloid sarcoma causing pericardial effusions makes this case unique. A 59-year-old patient presented to the emergency room with shortness of breath and was found to be in cardiac tamponade requiring urgent care. The thoracic surgery team performed a pericardiectomy and placed a window drain. Cardiac tissue biopsy proved cardiac myeloid sarcoma with immature blasts and myeloid cells. Initial bone marrow biopsy on admission showed hypercellularity with 19% blasts and abnormal monocytes with multilineage dysplasia on aspirate differential, with positive KRAS and TET2 mutations on next-generation sequencing analysis. There was no evidence of a BCR/ABL1 fusion on fluorescence in situ hybridization (FISH), and chromosomal analysis demonstrated a normal karyotype. At this time, the patient met the WHO criteria for a CMML-2 diagnosis. A follow-up bone marrow biopsy closer to discharge showed a 33% blast count pointing towards a CMML-2 transformation into AML. Remarkably, he was able to improve and was discharged from the hospital. He was admitted again to the hospital to initiate chemotherapy. Such complex cases are rarely reported. Here we discuss the diagnosis and treatment of CMML transformed into AML as well as the rarity of cardiac myeloid sarcomas with an in-depth literature review.
Keywords: Pericardial effusion; CMML; AML; Cardiac myeloid sarcoma
| Introduction | ▴Top |
Chronic myelomonocytic leukemia (CMML) is a clonal stem cell disorder with both myelodysplastic/myeloproliferative features defined by sustained monocytosis (> 1 × 109/L absolute count or > 10% of the leukocyte count), bone marrow dysplasia with less than 20% blasts, and the exclusion of other myeloid diseases based on genetics [1, 2]. CMML is a disease of elderly men with the average age of diagnosis around 70 years old and a male to female ratio of over 2:1 [3]. Patients with CMML can present with a wide variety of symptoms including severe cytopenias causing bleeding and infection, splenomegaly or, rarely, extramedullary invasion. Rare cases of CMML have been known to invade the gums, liver, or spleen and can present as an extramedullary collection of immature monocytes or dendritic cells. CMML presenting as a fluid collection in the pleura, pericardium or abdomen is an even more rare event [4-6].
Patients who are diagnosed with CMML, specifically, CMML-2 and myeloproliferative subtypes, are at a high risk of their disease transforming into acute myeloid leukemia (AML). Approximately 15-30% of all CMML cases will transform into AML with CMML-2 subtype being the highest risk and, rarely, AML can infiltrate other organs and present as myeloid sarcomas [7]. Myeloid sarcomas are extramedullary tumors that arise from the accumulation of blasts and other myeloid cells such as neutrophils or monocytes. They can appear in most places in the body, although cardiac infiltration is extremely rare. Myeloid sarcomas usually present concurrently with a new AML diagnosis or at relapse of an already established AML diagnosis and occur in roughly 5-10% of AML cases [8]. Primary myeloid sarcoma leading to an AML diagnosis accounts for < 2% of cases according to a case series from 2003 and has an incidence of two cases per million adults [9, 10].
Here, we present a case of CMML-2 transformed into AML with myeloid sarcoma involving the pericardium. This case highlights the unique presentation of myeloid sarcoma co-occurring with a CMML-2 diagnosis that was finally revealed after the patient had multiple pericardial effusions. We discuss the timeline of events, diagnostics, and treatment of this rare case. We also review CMML and myeloid sarcoma with an emphasis on diagnosis, treatment, and prevalence. Given the rarity of these diseases, this case offers a special opportunity to discuss both.
| Case Report | ▴Top |
Investigations
A 59-year-old male without significant past medical history had his first pericardial effusion identified in December of 2024 but was too small to drain. Notably, his complete blood count with differential back in September of 2018 revealed a monocytosis (1.5 × 103/µL) with no other abnormalities seen along with a normal blood smear. His next complete blood count with differential was done in December 2024 when he had his first pericardial effusion and it continued to show a monocytosis (7.2 × 103/µL) without blasts or other immature cells, but it was not until the following admission when further investigations were done. In February 2025, he presented to an outside hospital with shortness of breath on exertion and a syncopal episode. His echocardiogram on arrival to the hospital showed a new, large pericardial effusion with evidence of early tamponade and he underwent an uncomplicated pericardiocentesis with 700 cc of sanguineous output. He was also found to have splenomegaly with a complete blood count with differential showing a white blood cell count of 17.6 × 103/µL, platelet count of 112 × 103/µL, hemoglobin 13.3, and monocytosis of 8.61 × 103/µL. Once again, no blasts or other immature cells were seen in the differential and his blood smear was normal. The etiology of his pericardial effusion remained unclear at that time with cytology negative for any malignant cells, and he was discharged with outpatient follow-up with hematology for cytopenias and cardiology for serial echocardiograms. He had not yet seen the hematologist outpatient when soon after his February discharge, in early March 2025, he developed worsening shortness of breath with increasing pericardial effusion and signs of right ventricular collapse on echocardiogram. Another emergent pericardiocentesis yielded 250 cc of hemorrhagic fluid. This began a prolonged and complex hospital stay until early April of the same year. On arrival, a complete blood count with differential revealed a white blood cell count of 14.3 × 103/µL with persistent absolute monocytosis to 6.3 × 103/µL and worsening thrombocytopenia of 73 × 103/µL. Again, no blasts or other immature cells were seen at this time. After drainage, the patient subsequently had return of symptoms lasting a few days with associated resting tachycardia following placement of the pericardial drain. The drain had minimal output despite evidence of a reaccumulating complex pericardial effusion on bedside echocardiogram, so he was transferred to a tertiary care center for evaluation by thoracic surgery and consideration of a pericardial window. Upon transfer, he had a sudden decompensation due to obstructive shock secondary to cardiac tamponade. He underwent an emergent subxiphoid pericardial window placement complicated by significant clot burden in the pericardial sac. A few hours after this procedure, the patient decompensated again and his pericardial drain was found to be clotted off, so he returned to the operating room for complete pericardiectomy under cardiopulmonary bypass with mediastinal washout and removal of 200 cc of hemorrhagic fluid.
Diagnosis
The pericardial effusion samples from his hospital admissions were inflammatory and hemorrhagic in nature and did not reveal any leukemia cells. Given the patient’s abnormal cell lines with concerning monocytosis, thrombocytopenia, and anemia, he underwent his first bone marrow biopsy early during the March admission. This bone marrow biopsy showed hypercellularity with 19% blasts and abnormal monocytes with multilineage dysplasia on aspirate differential, with positive KRAS and TET2 mutations on next-generation sequencing analysis. Flow cytometry revealed CD34+ blasts at 1% with 28% monocytosis with CD56+ and no clonal or abnormal lymphoid populations. There was no evidence of a BCR/ABL1 fusion on fluorescence in situ hybridization (FISH), and chromosomal analysis demonstrated a normal karyotype. At this time, he met the 2016 WHO criteria for CMML-2 diagnosis by having a persistent monocytosis, blasts 5-19%, negative BCR/ABL with other myeloproliferative disorders ruled out, and the following genetic re-arrangements not detected: PDGFRA, PDGFRB, FGFR1, or PCM1-JAK2. He then subsequently underwent a biopsy of his pericardium after his bone marrow revealed CMML-2 with likely evolving AML. Figure 1 depicts the cardiac tissue sample used to diagnose cardiac myeloid sarcoma. Figure 2 shows cells staining positive for myeloperoxidase revealing the myeloblasts confirming leukemic involvement in the pericardium. The biopsy of his pericardium revealed findings consistent with myeloid sarcoma. A repeat bone marrow biopsy in early April during this same admission showed an increased manual differential count of blasts/promonocytes of 33% with multilineage dysplasia, consistent with CMML-2 transformation into AML. The monocyte count increased to 40% with aberrant CD14 marker expression per flow cytometry. Figure 3 shows myeloblasts present in the bone marrow. Figure 4 depicts the echocardiogram showing the large pericardial effusion causing tamponade and then its resolution following drainage.
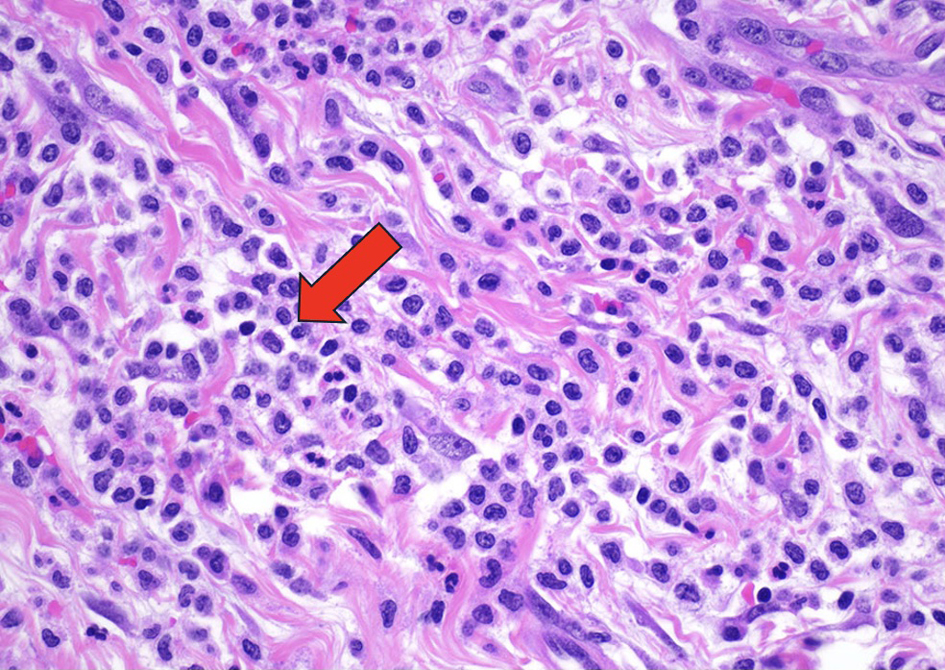 Click for large image | Figure 1. The myocardium and surrounding adipose tissue are architecturally effaced by an infiltrating lesion composed of immature mononuclear cells demonstrating fine chromatin. Hematoxylin and eosin (H&E)-stained sections of the pericardium demonstrated fibrovascular and fibroadipose tissue with a variably dense infiltration of medium sized cells with irregular nuclear membranes, conspicuous nucleoli, and variable amounts of clear cytoplasm, consistent with atypical/immature monocytic cells. These atypical cells formed diffuse sheets in some areas and were seen with large numbers of mature granulocytes in other areas. The immature cells demonstrated positive staining for CD45, CD43, CD34, CD68, and CD163. They were negative for CD117, TdT, CD3, CD20, CD56, calretinin, and WT1. The Ki-67 proliferation index was variably elevated. This atypical monocytic infiltration is diagnostic of myeloid sarcoma (H&E, × 400). Arrow indicating immature mononuclear cells infiltrating the cardiac tissue. |
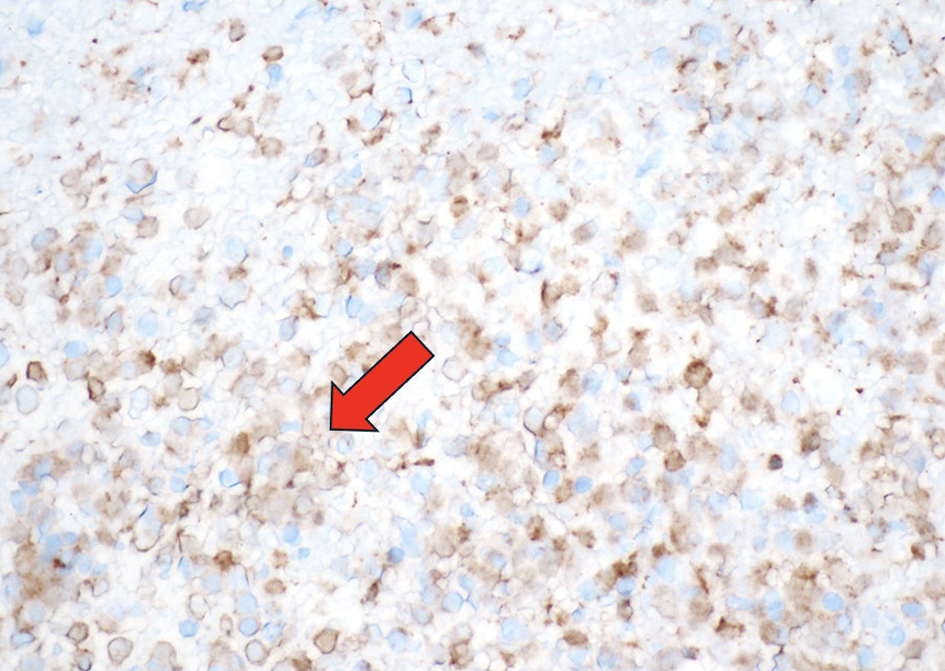 Click for large image | Figure 2. The immature mononuclear cells stain positive for myeloperoxidase, confirming the diagnosis of myeloid sarcoma (myeloperoxidase, × 400). Arrow indicating the light gold staining of the cells positive for myeloperoxidase. |
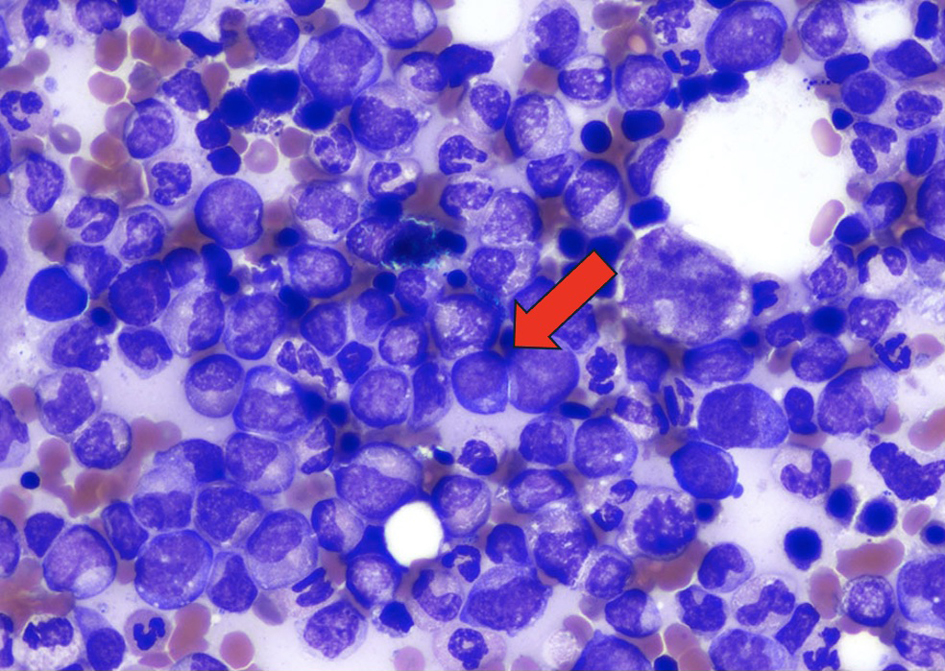 Click for large image | Figure 3. Wright Giemsa stain of the bone marrow demonstrating myeloblasts (× 1,000). Arrow indicating myeloblasts. |
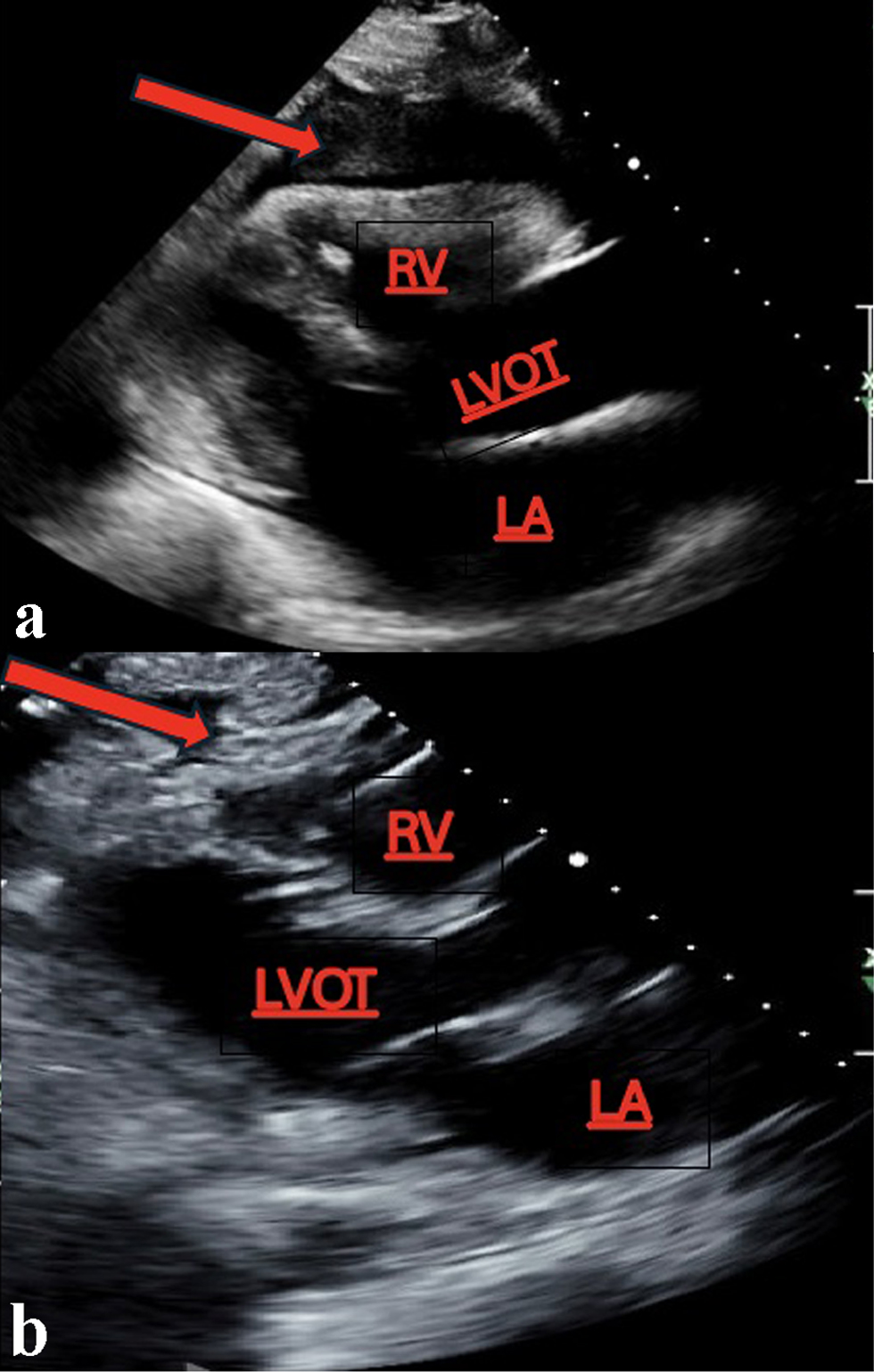 Click for large image | Figure 4. (a) A large pericardial effusion with RV collapse clinically correlating with cardiac tamponade. (b) Resolution of the pericardial effusion status after pericardiocentesis. Arrows indicating the fluid collection around the pericardium with RV collapse (a) with subsequent resolution after drainage (b). LA: left atrium; LVOT: left ventricular outflow tract; RV: right ventricle. |
Treatment and outcomes
Following his pericardiectomy, the patient’s cardiac management was stabilized in the cardiovascular intensive care unit (ICU). He required multiple transfusions of platelets and packed red blood cells, as well as 2 units of fresh frozen plasma (FFP). His subsequent inpatient stay was prolonged due to hypoxic respiratory failure and increasing oxygen requirements; imaging revealed large bilateral pleural effusions requiring chest tube placement yielding 1,350 cc of sanguinous fluid. Cytology from the pleural fluid was negative for leukemic involvement. The patient’s symptoms improved with aggressive diuresis, and he was successfully weaned to room air. His chest tube was removed, and he was discharged in stable condition on colchicine. Since the patient tolerated the pericardiectomy well, the decision was made to treat him as secondary AML ineligible for intensive 7+3 chemotherapy with understanding that he would be on the path for bone marrow transplant. The patient was started on decitabine 20 mg/m2 and venetoclax. A repeat bone marrow biopsy will be planned for week 3 of treatment. Further discussions surrounding transplant timing and feasibility would take place pending marrow results. Luckily, this patient survived after numerous days recovering in the cardiac ICU after pericardiectomy and pericardial window placement and is now living a remarkably normal life while starting treatment.
| Discussion | ▴Top |
CMML is an extremely rare disease with a reported incidence of four cases per 100,000 persons/year [11]. Many patients are asymptomatic at diagnosis and have only a mild monocytosis at presentation, but very few can present with inflammation of serous membranes with fluid accumulation around the pericardium or pleura. A 2021 retrospective study by Kaur et al looked at 9,723 cases of effusions in leukemic patients and only 40 (0.4%) showed true leukemic involvement. Of the 40 cases, only one was CMML and only three cases involved the pericardium highlighting the rarity of such an event [12]. Specifically, regarding acute leukemia, a 2010 retrospective study with 2,592 AML and acute lymphocytic leukemia (ALL) patients revealed a pericardial effusion incidence of 21%. However, very few of the samples actually had leukemia cells on cytology and most were hemorrhagic or serosanguinous [13]. There have been a few case reports published on CMML presenting as a pericardial effusion with positive cytology leading to confirmation with bone marrow biopsy [6, 14-17]. However, having CMML transform into AML and then present as a cardiac myeloid sarcoma causing recurrent pericardial effusions represents a unique case.
Due to the rarity of cardiac myeloid sarcomas, most information we have on them is through case reports or case series and no large-scale prospective studies exist that give information on survival. A review of cardiac myeloid sarcomas done in 2017 compiled all published cases of adult and pediatric cardiac myeloid sarcomas from 1960 to 2016 and found only 30 with six of these patients achieving remission [18]. However, none of these patients had a confirmed CMML diagnosis. There is no consensus on how to treat cardiac myeloid sarcomas and hematologists will need to consider tolerability and cardiac function when deciding on a regimen to use. Anthracyclines are a standard of treatment in AML, but given their cardiac toxicities, may not be suitable in these cases. Intensive induction chemotherapy regimens such as etoposide, mitoxantrone, and cytarabine/Ara-C-based regimens (EMA) as well as hypomethylating agents (HMAs) have been used according to a few case reports. One case report highlights a patient who achieved remission after their cardiac myeloid sarcoma was treated with radiotherapy [19].
Managing myeloproliferative CMML can be difficult and those with transformed disease ineligible for intensive chemotherapy are treated with HMA with or without venetoclax based on the results of the VIALE-A trial [20]. Initially, clinical trials involving myelodysplasia (MDS) patients that confirmed the efficacy of azacitidine and decitabine included very few CMML patients [21, 22], but smaller follow-up studies focusing on CMML showed an overall response rate between 30% and 60% with complete remission in less than 15% of patients [23-25]. Median overall survival (OS) ranged between 12 and 37 months. Patients with CMML who fail a first-line HMA have little success when switched to a different agent [26]. The DACOTA trial was a randomized phase III trial testing decitabine or hydroxyurea in CMML patients. While 63% of patients in the decitabine arm had a response vs. only 35% in the hydroxyurea cohort, treatment with decitabine did not improve event-free survival and had a slightly lower median overall survival (mOS) (18.4 vs. 21.9 months) [27]. This seems to be in contrast with a 2021 study showing modest survival benefit with decitabine vs. hydroxyurea (mOS 17.6 vs. 12.6 months) [28]. Regardless, because of the high response rate seen in the decitabine cohort, this could be a better choice for patients as a bridge to transplant. More randomized trials with adequate sample size are needed in rare leukemias such as CMML to better map out treatment algorithms. The patient presented in this case had a complex disease history with CMML turned AML presenting as a cardiac myeloid sarcoma and there is no standard of treatment for such a complicated case.
Conclusion
CMML presenting as a myeloid sarcoma in the pericardium is a rare and deadly phenomenon that needs urgent action to prevent hemodynamic collapse from tamponade caused by pericardial effusion. The insidious progression of CMML-2 in this patient caused a complex hospital stay which ultimately led to a successful pericardiectomy. This case highlights the challenge of diagnosing and recognizing CMML. He had a persistent, mild monocytosis for many years before presenting with his first pericardial effusion and no other warning signs were present. For example, no immature cells on lab work, fevers, fatigue or weight loss were seen. Unfortunately, his negative symptoms and, initially, only mild monocytosis caused a delay in the diagnosis of the CMML that eventually evolved into acute leukemia with extramedullary involvement in the form of a myeloid sarcoma. When his symptoms worsened with shortness of breath, repeat complete blood counts revealed not only a significant increase in monocytosis, but also thrombocytopenia and anemia prompting the initial bone marrow biopsy leading to the CMML diagnosis during his hospital admission. Earlier referral to a hematologist for surveillance and shared decision making regarding an elective bone marrow biopsy with further laboratory work may have identified this disease earlier. However, the swift treatment and multidisciplinary teamwork of the hematology, critical care, and cardiology teams led to a positive outcome. With such a complex and life-threatening disease, multidisciplinary teamwork and high resource centers are key to success.
Learning points
CMML transformed into AML presenting as a myeloid sarcoma in the pericardium has never been published before.
CMML is hard to diagnose and treat while sometimes presenting as a slow growing, mild monocytosis without any symptoms.
Standard treatment for CMML involves cytoreduction and combination therapy with an HMA and venetoclax.
Cardiac myeloid sarcomas are extremely rare with limited information on treatment and outcomes.
Acknowledgments
None to declare.
Financial Disclosure
The authors have no funding source to disclose for this case report.
Conflict of Interest
The authors have no conflict of interest.
Informed Consent
Not applicable, for without individual identifiable information.
Author Contributions
Austin Frisch: original draft preparation with literature review and figure creation (lead). Rohan Boyapati and Ruja Parikh: draft preparation and case section. Geetha Menezes: review and editing. Niharika Tipirneni, Germame Ajebo, and Danielle Shafer: review, editing, and visualization of concept.
Data Availability
The authors declare that the data supporting the findings of this study are available within the article.
| References | ▴Top |
- Kwon J. Diagnosis and treatment of chronic myelomonocytic leukemia. Blood Res. 2021;56(S1):S5-S16.
doi pubmed - Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405.
doi pubmed - Marando L, Csizmar CM, Patnaik MM. Chronic myelomonocytic leukemia: molecular pathogenesis and therapeutic innovations. Haematologica. 2025;110(1):22-36.
doi pubmed - Itzykson R, Fenaux P, Bowen D, Cross NCP, Cortes J, De Witte T, Germing U, et al. Diagnosis and treatment of chronic myelomonocytic leukemias in adults: recommendations from the European Hematology Association and the European LeukemiaNet. Hemasphere. 2018;2(6):e150.
doi pubmed - Morita Y, Ohyama Y, Rai S, Kawauchi M, Yamaguchi T, Shimada T, Tatsumi Y, et al. A case of chronic myelomonocytic leukemia who developed pericardial effusion during stably controlled leukocytosis. Intern Med. 2011;50(16):1737-1740.
doi pubmed - Hu L, Zheng B, Fu L, Hu M. Chronic myelomonocytic leukemia (CMML)-0 with pleural effusion as first manifestation: A case report. Medicine (Baltimore). 2020;99(44):e23030.
doi pubmed - Geissler K. Molecular pathogenesis of chronic myelomonocytic leukemia and potential molecular targets for treatment approaches. Front Oncol. 2021;11:751668.
doi pubmed - Kuhlman JJ, Abdel Rahman ZH, Jiang L, Menke DM, Foran JM, Murthy HS. Primary peritoneal myeloid sarcoma in association with CBFB/MYH11 fusion. Leuk Res Rep. 2021;15:100238.
doi pubmed - Tsimberidou AM, Kantarjian HM, Estey E, Cortes JE, Verstovsek S, Faderl S, Thomas DA, et al. Outcome in patients with nonleukemic granulocytic sarcoma treated with chemotherapy with or without radiotherapy. Leukemia. 2003;17(6):1100-1103.
doi pubmed - Magdy M, Abdel Karim N, Eldessouki I, Gaber O, Rahouma M, Ghareeb M. Myeloid sarcoma. Oncol Res Treat. 2019;42(4):224-229.
doi pubmed - El Hussein S, Wang SA, Pemmaraju N, Khoury JD, Loghavi S. Chronic myelomonocytic leukemia: hematopathology perspective. J Immunother Precis Oncol. 2021;4(3):142-149.
doi pubmed - Kaur K, Patel T, Patra S, Trivedi P. Cytomorphology, Immunophenotype, and cytogenetic profile of leukemic serous effusions. Diagn Cytopathol. 2021;49(8):948-958.
doi pubmed - Sampat K, Rossi A, Garcia-Gutierrez V, Cortes J, Pierce S, Kantarjian H, Garcia-Manero G. Characteristics of pericardial effusions in patients with leukemia. Cancer. 2010;116(10):2366-2371.
doi pubmed - Strupp C, Germing U, Trommer I, Gattermann N, Aul C. Pericardial effusion in chronic myelomonocytic leukemia (CMML): a case report and review of the literature. Leuk Res. 2000;24(12):1059-1062.
doi pubmed - Yufu Y, Okada Y, Goto T, Nishimura J. Cardiac tamponade in chronic myelomonocytic leukemia: a case report. Jpn J Clin Oncol. 1992;22(6):411-413.
pubmed - Ibrahim M, Maslak P, Heaney M, George M, Downey R, Tallman M. Chronic myelomonocytic leukemia (CMML) associated with symptomatic pericardial effusion. Journal of Clinical Oncology. 2011;29(15_suppl):6625.
- Wauye VM, Njiru E, Amadi AK, Hagembe MN, Kigen G. Chronic myelomonocytic leukemia primarily presenting as life-threatening pericardial effusion, Eldoret, Kenya: A case report. Clin Case Rep. 2024;12(6):e9048.
doi pubmed - Gautam A, Jalali GK, Sahu KK, Deo P, Ailawadhi S. Cardiac myeloid sarcoma: review of literature. J Clin Diagn Res. 2017;11(3):XE01-XE04.
doi pubmed - Yang WC, Yao M, Chen YH, Kuo SH. Complete response of myeloid sarcoma with cardiac involvement to radiotherapy. J Thorac Dis. 2016;8(6):1323-1328.
doi pubmed - DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, Konopleva M, et al. Azacitidine and Venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629.
doi pubmed - Kantarjian H, Oki Y, Garcia-Manero G, Huang X, O'Brien S, Cortes J, Faderl S, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109(1):52-57.
doi pubmed - Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, Schoch R, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223-232.
doi pubmed - Pleyer L, Germing U, Sperr WR, Linkesch W, Burgstaller S, Stauder R, Girschikofsky M, et al. Azacitidine in CMML: matched-pair analyses of daily-life patients reveal modest effects on clinical course and survival. Leuk Res. 2014;38(4):475-483.
doi pubmed - Drummond MW, Pocock C, Boissinot M, Mills J, Brown J, Cauchy P, Cross NC, et al. A multi-centre phase 2 study of azacitidine in chronic myelomonocytic leukaemia. Leukemia. 2014;28(7):1570-1572.
doi pubmed - Tantravahi SK, Szankasi P, Khorashad JS, Dao KH, Kovacsovics T, Kelley TW, Deininger MW. A phase II study of the efficacy, safety, and determinants of response to 5-azacitidine (Vidaza(R)) in patients with chronic myelomonocytic leukemia. Leuk Lymphoma. 2016;57(10):2441-2444.
doi pubmed - Harel S, Cherait A, Berthon C, Willekens C, Park S, Rigal M, Brechignac S, et al. Outcome of patients with high risk Myelodysplastic Syndrome (MDS) and advanced Chronic Myelomonocytic Leukemia (CMML) treated with decitabine after azacitidine failure. Leuk Res. 2015;39(5):501-504.
doi pubmed - Itzykson R, Santini V, Thepot S, Ades L, Chaffaut C, Giagounidis A, Morabito M, et al. Decitabine versus hydroxyurea for advanced proliferative chronic myelomonocytic leukemia: results of a randomized phase III trial within the EMSCO network. J Clin Oncol. 2023;41(10):1888-1897.
doi pubmed - Pleyer L, Leisch M, Kourakli A, Padron E, Maciejewski JP, Xicoy Cirici B, Kaivers J, et al. Outcomes of patients with chronic myelomonocytic leukaemia treated with non-curative therapies: a retrospective cohort study. Lancet Haematol. 2021;8(2):e135-e148.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.