| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com |
Case Report
Volume 000, Number 000, June 2025, pages 000-000
An Unusual Cause of Neonatal Infection: A Case Report of Campylobacter coli Meningitis and Sepsis
Dimitrios Kapnisisa , Christodoulos Chatzigrigoriadisb, i
, Emmanouil Koufopoulosb
, Fevronia Kolonitsiouc
, Gabriel Dimitrioua
, Sotirios Fouzasa
, Panagiotis Eskitzisd
, Lazaros Lavasidisd
, Doxakis Anestakise, f
, Despoina Sperdoulig
, Panagis Galiatsatosh
, Despoina Gkentzia
aDepartment of Pediatrics, University General Hospital of Patras, Patras Medical School, Patras, Greece
bSchool of Medicine, University of Patras, Patras, Greece
cDepartment of Microbiology, University General Hospital of Patras, Patras Medical School, Patras, Greece
dDepartment of Midwifery, School of Healthcare Sciences, University of Western Macedonia, Keptse 50200, Ptolemaida, Greece
eDepartment of Pathology-Forensic Sciences, Medical School, University of Cyprus, Nicosia, Cyprus
fDepartment of Pathology, Forensic Service of Thessaloniki, Ministry of Justice, Thessaloniki, Greece
gSchool of Medicine, National Kapodistrian University of Athens, Athens, Greece
hDepartment of Internal Medicine, Johns Hopkins University School of Medicine, Baltimore, MD 21224, USA
iCorresponding Author: Christodoulos Chatzigrigoriadis, School of Medicine, University of Patras, Patras, Greece. Εmail:
Manuscript submitted April 22, 2025, accepted May 29, 2025, published online June 16, 2025
Short title: Campylobacter coli Neonatal Meningitis
doi: https://doi.org/10.14740/jmc5133
| Abstract | ▴Top |
Neonatal meningitis is associated with increased morbidity, mortality, and long-term consequences. Despite the use of newer techniques, the diagnosis remains challenging, especially in cases caused by rare pathogens. Campylobacter is widely known as the most common cause of bacterial gastroenteritis. However, invasive infections in neonates have been rarely described in the literature. A rare case of neonatal meningitis caused by Campylobacter coli is presented in this case report. A 14-day-old male and late preterm neonate without a remarkable perinatal history was admitted to our Pediatric Department with a 10-h history of fever and loose stools. The initial laboratory studies suggested the diagnosis of meningitis, but isolating the responsible pathogen in blood and cerebrospinal fluid cultures was demanding. After the cultures were repeated and incubated in microaerophilic conditions, Campylobacter coli was confirmed as the etiological agent. Based on antibiotic susceptibility tests, the neonate had a 21-day course of antibiotic therapy with cefotaxime, a third-generation cephalosporin, and remained healthy during the illness without experiencing any neurological sequelae. This case report highlights that rare pathogens should be considered and searched for in cases of neonatal meningitis when there is no identifiable cause with routine microbiological techniques.
Keywords: Campylobacter; Campylobacter coli; Neonate; Meningitis; Sepsis; Diarrhea; Infection; Gastroenteritis
| Introduction | ▴Top |
Sepsis and meningitis are life-threatening emergencies in neonates. Neonatal meningitis increases morbidity and mortality [1]. Although the cases of neonatal meningitis have decreased in the last five decades, the diagnosis is demanding in cases caused by rare pathogens [2]. The incidence of bacterial meningitis in neonates varies from 0.25 and 0.32 per 1,000 live births [2]. The prevalence of bacterial meningitis in neonates with bacteremia is estimated to be 15% [2]. The diagnosis is mainly clinical but confirmed by blood, urine, and cerebrospinal fluid (CSF) cultures. The empiric antibiotic regimens of choice are: ampicillin plus cefotaxime, ampicillin plus gentamicin, or ampicillin plus cefotaxime plus gentamicin. These combinations cover the most common causes: group B Streptococcus (GBS), Enterobacteriaceae (mainly Escherichia coli), and Listeria monocytogenes [2-4]. A minority of neonatal infections are caused by Campylobacter species [4].
Campylobacter spp are comma-shaped, gram-negative rods biochemically positive for oxidase and catalase [5, 6]. It is widely known as the most common cause of bacterial diarrhea [5, 7-12]. More than 10 species can cause human infection; C. jejuni and C. coli are the most common, but this is questioned in developing countries [5, 7, 13]. C. fetus is known for its vascular tropism [5, 11, 14-16]. The gastrointestinal (GI) tract of various animals (especially birds) is a reservoir for transmission to humans [5]. Thus, its transmission is mostly foodborne; meat consumption is the most common risk factor, especially poorly cooked poultry [5-7, 17]. However, Campylobacter spp rarely affect fetuses or neonates; a maternal genital tract or systemic infection can spread to the fetus, while transmission to the neonate occurs during birth or an outbreak [5, 18-22].
Campylobacter spp colonize the GI tract causing a variable clinical presentation ranging from asymptomatic carriage to dysentery after an incubation period of 1 to 7 days (average 3 days) [5]. Stool testing and antibiotic treatment are sometimes necessary for complicated cases [5, 17, 23-25]. The requirements for microaerobic conditions, the prolonged incubation period, and the ability of Campylobacter spp to form viable but non-culturable cells (VBNCs) contribute to the limited sensitivity of the cultures [6-8, 14]. Occasionally, Campylobacter spp enter the bloodstream in high-risk patients causing bacteremia and/or localized infections, but these complications also occur in young and healthy patients [5, 6, 10, 11, 14-21, 26-29] (Table 1 and Fig. 1). The occurrence of Campylobacter bacteremia depends on either the host or the pathogen [14] (Table 2).
 Click to view | Table 1. Extraintestinal Complications of Campylobacter Infection on Various Systems |
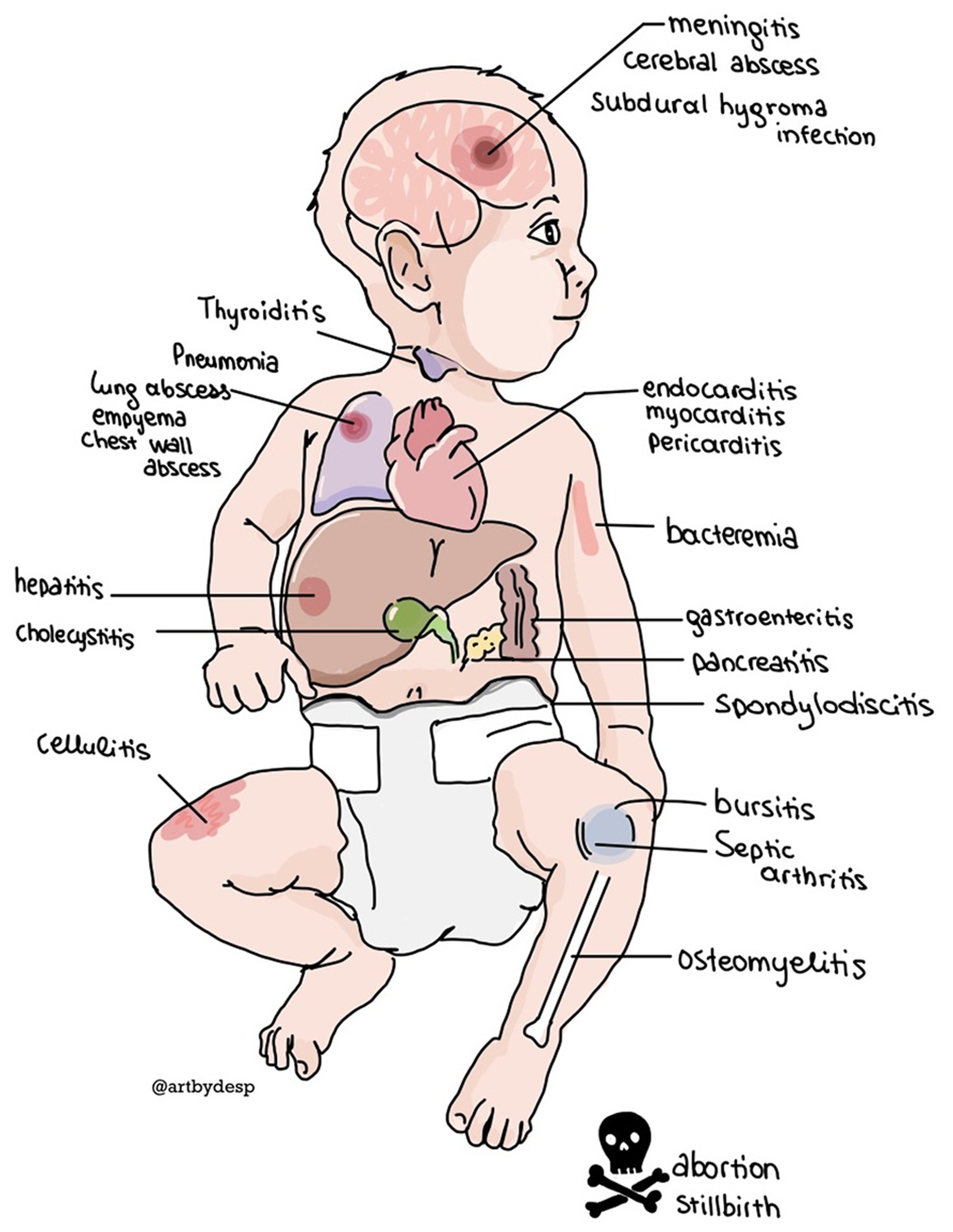 Click for large image | Figure 1. The extraintestinal locations of disseminated Campylobacter infection. |
 Click to view | Table 2. Risk Factors for the Disseminated Campylobacter Infection |
Limited evidence is available for the appropriate management of Campylobacter bacteremia and meningitis. Cultures or molecular tests on blood and CSF establish the diagnosis. Although the management of intestinal infections involves supportive care or the administration of antibiotics, such as macrolides and fluoroquinolones, the safest choice for invasive infections is prolonged treatment with intravenous carbapenems or aminoglycosides [4, 5, 16, 27, 30, 31]. Additional treatment with oral antibiotics may be considered [4, 16, 30, 31].
Herein, we report a rare case of Campylobacter coli meningitis in a neonate, hospitalized in our Pediatric Department.
| Case Report | ▴Top |
A previously healthy 14-day-old male and late preterm neonate, the first baby of a healthy, unrelated couple, was admitted to our Pediatric Department with a 10-h history of fever and loose stools. Perinatal history revealed premature rupture of membranes. Parents reported no GI symptoms and denied any contact with poultry. The neonate was bottle-fed with formula. Physical examination of the baby was unremarkable. Laboratory evaluation did not reveal elevated inflammatory markers (white blood cells (WBCs): 25,030/µL (normal values 9,000 - 30,000/µL), neutrophils (NEUT): 63.9%, lymphocytes (LYMPH): 21.3%, and C-reactive protein (CRP): 0.36 mg/dL (normal value < 0.5 mg/dL)). A lumbar puncture revealed pleocytosis (WBC: 7,200/µL (normal range 0 - 29), 95% polynuclear type), elevated CSF protein 196 mg/dL (normal values 65 - 170 mg/dL), and slightly low CSF glucose (39 mg/dL) (normal values 24 - 63 mg/dL). Empiric antibiotic therapy with ampicillin, cefotaxime, and gentamycin was initiated. The FilmArray meningitis/encephalitis panel of CSF was negative. On day 3, a gram-negative, oxidase-positive, and S-shaped bacterium was detected in blood and CSF cultures, which were then both repeated and incubated microaerophilically at 42 °C for 72 h. Heavy growth was detected on laboratory media at 42 °C, which was identified as Campylobacter coli (VITEK® 2, bioMerieux) (Fig. 2). The neonate completed a 7-day course with gentamycin, a 10-day course with ampicillin, and a 21-day course of cefotaxime. The prolonged course of cefotaxime was based on antibiotic susceptibility testing. Azithromycin was added for 5 days for the sterilization of the GI tract. The neonate was febrile for 2 days after admission. The maximum CRP value was 1.07 mg/dL. A lumbar puncture was repeated after completing the antibiotic course without abnormal findings (WBC: 17/µL, NEUT: 3/μL, LYMPH: 6/μL, CSF protein: 70.4 mg/dL). The baby remained well throughout the disease with satisfactory weight gain, and no neurological sequelae were identified. The 6-month follow-up assessment revealed normal clinical, neurological, and developmental examinations.
 Click for large image | Figure 2. Colonies on blood agar, incubated at 37 °C (left) and microaerophilically at 42 °C (right) revealed the microaerophilic optimal growth of Campylobacter spp (Microbiology Laboratory, University General Hospital of Patras). |
| Discussion | ▴Top |
Neonatal infection with Campylobacter spp is usually acquired during birth from mothers who excrete the bacteria in the stool or because of small family enteritis outbreaks, but none of these hypotheses were confirmed in our case. Campylobacter spp colonize the GI tract of many animals and are excreted in their feces [5, 32, 33]. Thus, transmission is mostly foodborne; Campylobacter infection is linked with meat consumption, especially poorly cooked poultry [5, 7, 17, 32, 33] (Fig. 3). Contact with animals and their feces is associated with Campylobacter transmission, especially in the developing world [5, 32]. Hence, hand hygiene is essential to prevent campylobacteriosis [5, 32]. Most reports of Campylobacter gastroenteritis are sporadic, but outbreaks associated with contaminated water or milk occasionally occur [5, 17, 32]. In developed countries, the age distribution of Campylobacter gastroenteritis is bimodal; most cases are observed in patients younger than 5 years of age and young adults [5]. On the contrary, most cases of Campylobacter gastroenteritis in developing countries occur during the first 5 years [5]. Fetal or neonatal transmission is rarely reported in the literature; thus, the details of the transmission are unknown [5] (Fig. 3). Cases of abortion, intrauterine fetal demise, and prematurity were associated with maternal Campylobacter infection in the genital organs and extragenital locations [19-21]. Sexual transmission of Campylobacter spp is established in cattle and men who have sex with men; thus, male-to-female transmission is thought to be possible, but further research is required [5, 19-21]. It is speculated that extragenital Campylobacter infections, such as gastroenteritis and pneumonia, spread to the fetus through the bloodstream [20]. It remains unclear if Campylobacter spp spread from the genital tract to the fetus directly or indirectly through the bloodstream. Neonatal transmission is believed to occur perinatally or during the hospital stay [5, 18-22]. It is hypothesized that the excretion of Campylobacter spp in maternal feces leads to contamination of the perineum and the genital area and then spreads to the neonate perinatally [5, 19, 20]. Multiple factors are hypothesized to contribute to the development of outbreaks in neonatal units: asymptomatic carriage in neonates and healthcare providers, sharing of medical devices without proper disinfection, use of nasogastric tubes, and poor hand hygiene [18, 22]. The neonate in this case was probably infected perinatally, but no source of infection was found.
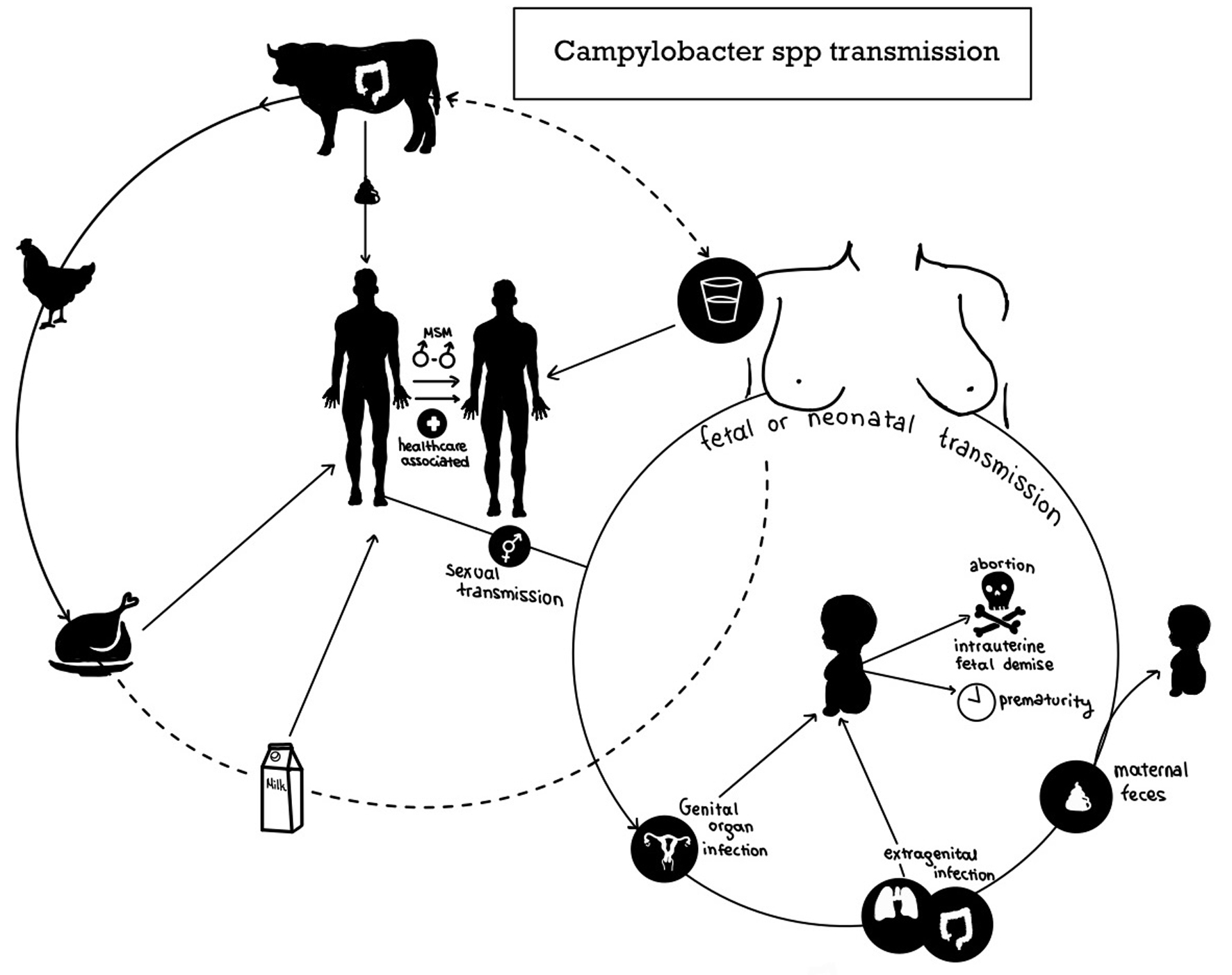 Click for large image | Figure 3. The transmission routes of Campylobacter spp to human beings, including fetuses and neonates. |
The clinical presentation of campylobacteriosis is highly variable (Fig. 4). Intestinal involvement is the most common site; asymptomatic infection or acute infectious diarrhea are the main forms of Campylobacter infection 1 - 7 days after exposure (average 3 days) [5, 18]. C. jejuni and C. coli are more likely to cause dysentery than other species [13]. GI carriage of Campylobacter spp is likely to be asymptomatic in patients immunized by previous exposure [5]. Asymptomatic excretion of Campylobacter spp in feces is also observed after an episode of gastroenteritis for 2 - 3 weeks, but sometimes the clearance is delayed for months [5]. Poor linear growth is associated with prolonged asymptomatic GI carriage of Campylobacter spp [13]. The intestinal symptoms are usually mild and non-specific, including nausea, vomiting, abdominal pain, fever, and watery or bloody diarrhea lasting 1 - 2 days [5]. Certain criteria differentiate between uncomplicated and complicated Campylobacter infections, guiding the management [5, 6, 10, 11, 16, 17, 23] (Table 3). Complicated cases pose a higher risk of dehydration or sepsis, requiring proper diagnostic testing and treatment [5, 17, 23]. Although all neonatal Campylobacter infections are considered complicated, most neonates develop self-limited gastroenteritis [18, 22]. In our case, the GI tract could be a possible route of infection, although this was not confirmed.
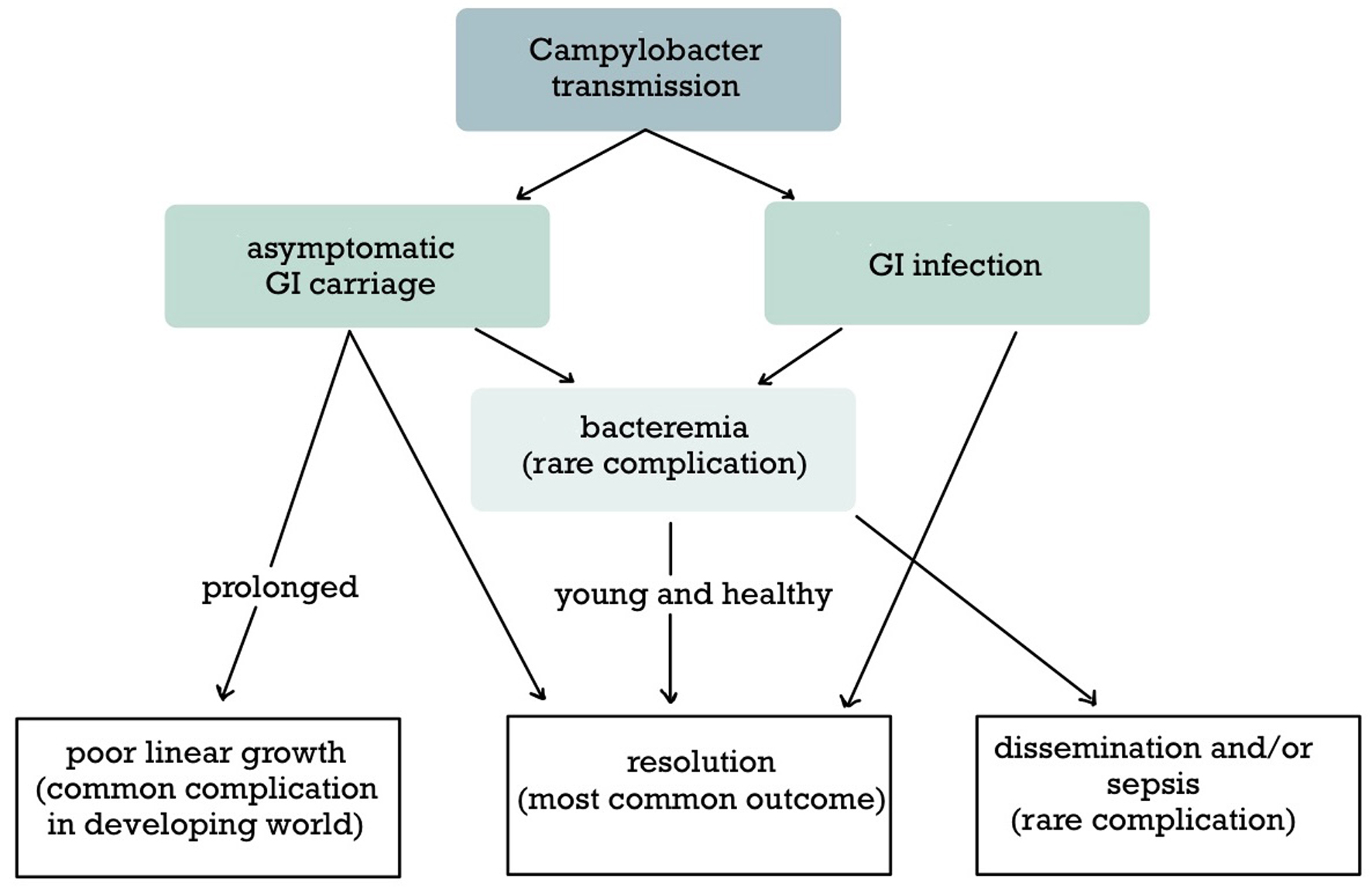 Click for large image | Figure 4. The clinical course of Campylobacter infection. Campylobacter infection is either symptomatic or asymptomatic and usually remains in the gastrointestinal tract. Dissemination to the bloodstream and other extraintestinal sites is rare. An important outcome in the developing world is prolonged asymptomatic gastrointestinal carriage leading to malnutrition. |
 Click to view | Table 3. Classification of Campylobacter Infection Depends on the Presence of Complications and the Location (Intestinal Versus Extraintestinal) |
Extraintestinal complications, such as bacteremia and meningitis, are rare; bacteremia complicates less than 1% of the total cases of campylobacteriosis [6, 9, 16]. Although controversial, bacteremia usually occurs in high-risk patients, such as patients with malignancies, immunodeficiencies, and extreme prematurity [5, 12, 16, 21, 34, 35]. Pre-existing brain pathology, immunocompromised status, and neonatal age predispose to Campylobacter central nervous system (CNS) infection [26, 27]. It should be noted that it is difficult to differentiate clinically Campylobacter bacteremia from Campylobacter gastroenteritis [34]. Interestingly, diarrhea is less prominent in bacteremic patients [12, 21]. The clinical presentation of Campylobacter meningitis is like other causes of acute bacterial meningitis, while intestinal involvement may be present [26, 27]. Notably, meningitis should be differentiated from meningismus and febrile seizures in the context of Campylobacter intestinal infection [5, 19]. The prognosis of Campylobacter bacteremia is highly variable and depends on the comorbidities, but it seems better than bacteremia caused by other species [11]. Self-limited bacteremia has been reported in many otherwise healthy patients, including pediatric patients [11, 12, 15]. However, Campylobacter bacteremia tends to recur in patients with impaired immunity. For example, patients with Bruton agammaglobulinemia (X-linked agammaglobulinemia) are especially susceptible to Campylobacter infection due to the loss of the protective role of serum immunoglobulin M (IgM) and mucosal immunoglobulin A (IgA) despite the repletion of immunoglobulin G (IgG) after the administration of intravenous immunoglobulin (IVIG) [10, 31]. Few data are available for localized extraintestinal complications without bacteremia, e.g., meningitis, but their outcome may be worse than isolated bacteremia [14]. The prognosis of Campylobacter meningitis in neonates is also controversial due to the limited number of cases [19, 27].
The diagnosis of campylobacteriosis is primarily microbiological given its non-specific clinical presentation [5] (Fig. 5). Although the recommendations of the Infectious Diseases Society of America (IDSA), the American College of Gastroenterology (ACG), the American Academy of Pediatrics (AAP), and the Association of the Military Surgeons of the United States (AMSUS) slightly differ, the indications for stool testing are established [5, 17, 23, 24] (Table 4). Stool culture is the gold-standard diagnostic method for intestinal Campylobacter infection, although culture-independent techniques (CIDTs), such as nucleic acid amplification test (NAAT) and immunochromatographic tests (ICTs) are promising [5-8, 14, 17, 23, 24, 26] (Table 5). Repeating stool testing can increase the diagnostic yield [23]. Campylobacter bacteremia is an unusual complication, but IDSA and AAP recommend obtaining blood cultures in the presence of certain indications [5, 17] (Table 4). When the clinical presentation is consistent with a localized extraintestinal complication, e.g., meningitis, cultures should also be obtained from appropriate sites, e.g., CSF culture [26]. In this case, the cultures mentioned above were obtained because it was a febrile neonatal infection.
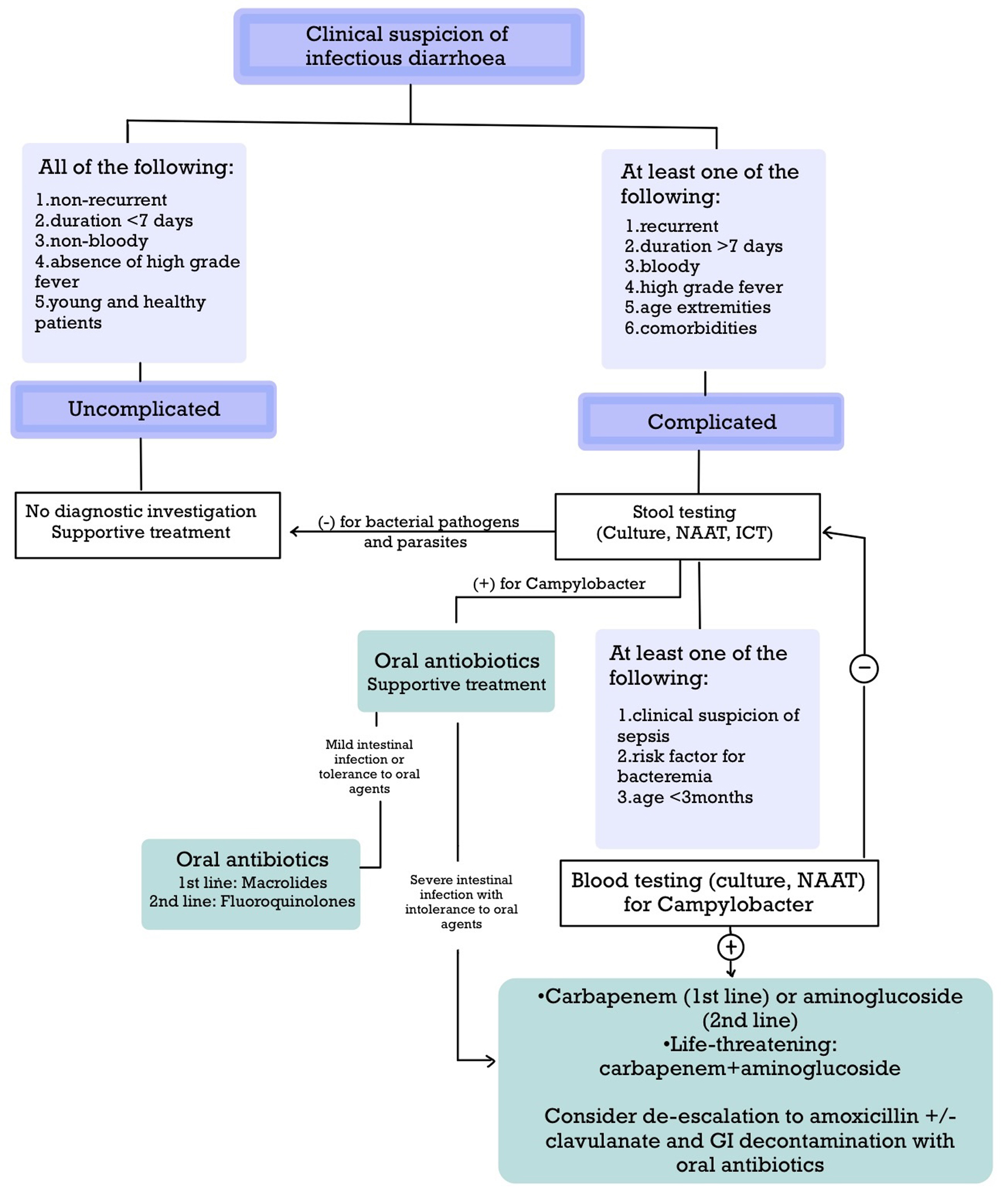 Click for large image | Figure 5. Approach to infectious diarrhea with special considerations for diagnosing and treating Campylobacter infection. |
 Click to view | Table 4. Stool With or Without Blood Testing for Enteropathogens Is Indicated in a Minority of Patients With Infectious Diarrhea |
 Click to view | Table 5. A Comparison of the Diagnostic Techniques (Cultures, NAAT, ICT) for Detecting Campylobacter spp. Combining a CIDT With Culture is Generally Recommended |
Campylobacter spp are oxidase/catalase-positive, comma-shaped or S-shaped gram-negative pathogens [5, 6]. The growth of Campylobacter spp is slow (48 - 72 h), although more time may be necessary for species other than C. jejuni, C. coli, and C. lari [6-8]. The organisms are easily identified in the routine processing of stool samples, but their detection is difficult in blood and CSF cultures without microaerophilic incubation [35]. A medium with selective antibiotics and microaerophilic conditions (5-10% oxygen, 1-10% carbon dioxide, and some hydrogen) is necessary [6, 7, 14]. The thermophilic species of Campylobacter jejuni/coli grow ideally at 42 °C in the presence of cephalothin, although growth has been observed at 37 °C [5-7]. The ideal temperature for culturing Campylobacter non-jejuni/coli is 37 °C [7]. Regarding basal blood agar media, Boston and Columbia’s blood agar media are the most effective ones [7]. Furthermore, the Boston blood agar was slightly more effective than Columbia agar for the detection of 16 Campylobacter species when combined with antibiotics; the Columbia blood agar was the best choice for culturing the four main species (C. jejuni, C. coli, C. lari, and C. fetus) in contrast to the Boston blood agar for the other 12 species [7]. C. jejuni is differentiated from other species (including C. coli) by a positive hippurate hydrolysis test [6]. Culture results should include information about the species, the strain, and the antibiotic susceptibility profile, which guides the antibiotic treatment according to the IDSA, AAP, and AMSUS [5, 17, 24]. However, the ACG recommends testing for antibiotic susceptibility only for epidemiologic purposes and in the setting of outbreaks [23].
NAATs are more sensitive tests than cultures (especially in unsatisfactory samples) that provide faster results within hours [5-8, 17, 23, 26]. This is essential because it allows rapid administration of antibiotics maximizing their efficacy. Multiplex polymerase chain reaction (PCR) can test multiple pathogens simultaneously [17, 23, 24]. However, NAATs detect nucleic acids instead of pathogenic microorganisms, which explains the higher rate of false-positive results due to the asymptomatic carriage of enteropathogens [5, 17, 23, 24]. Consequently, detecting multiple pathogens often complicates the interpretation and management [5, 17, 23, 24]. The lack of specificity and information about antibiotic susceptibility limits the clinical usefulness of molecular testing [5, 17, 23, 24]. Thus, a reflex culture is generally recommended after a positive molecular test [5, 17]. The ICT is a novel technique to detect Campylobacter spp in stool specimens [8]. It is much faster and easier to use than molecular methods because the results are available within minutes, and automation is unnecessary. Interestingly, ICT appears more sensitive than cultures in detecting C. jejuni/coli but lacks sensitivity for detecting other species. Further research is necessary to establish the diagnostic role of ICT relative to NAAT and cultures in future guidelines.
Extraintestinal Campylobacter infection poses a diagnostic challenge for clinicians [4, 10-12, 14, 19] (Fig. 5). Blood and CSF cultures are essential for diagnosing Campylobacter bacteremia and meningitis, and molecular methods also apply to blood and CSF specimens [5-7, 11, 14, 17, 26]. However, Campylobacter bacteremia as well as other extra-intestinal complications is believed to be underreported for various reasons; lack of collection of blood cultures in patients with infectious diarrhea despite the presence of fever, delayed sampling or processing, and the growth of Campylobacter requires special conditions and a more extended incubation period [9, 12, 14, 35]. A lower volume of blood samples may contribute to the underdiagnosis of Campylobacter bacteremia in children and neonates. Subculturing in solid media is a method to detect undiagnosed Campylobacter bacteremia after 5 - 7 days of negative results in standard cultures [14]. A multidisciplinary collaboration is necessary to identify Campylobacter spp in extraintestinal sites. In this case, isolating Campylobacter coli from blood and CSF cultures was challenging following incubation under microaerophilic conditions at 42 °C.
Campylobacter gastroenteritis is the most common manifestation of Campylobacter infection; its treatment is well-established (Table 3 and Fig. 5). Most patients develop uncomplicated intestinal infections requiring only symptomatic treatment with repletion of fluids/electrolytes and administering antiemetics/analgesics [5, 22, 30]. A minority of patients require inpatient care [5]. The role of probiotics for viral and antibiotic-associated diarrhea is well-established; they may also be effective in bacterial diarrhea but are contraindicated in patients with critical illness and impaired immunity [5, 17, 23]. Zinc supplements are recommended for patients aged from 6 months to 5 years living in areas with a high prevalence of zinc deficiency or malnutrition [5].
Antibiotic treatment is indicated in patients with complicated Campylobacter gastroenteritis [5, 6, 10, 11, 16, 17] (Table 3 and Fig. 5). Antibiotics, such as macrolides and fluoroquinolones, shorten the duration of Campylobacter-induced diarrhea; the benefit is maximized in patients who receive antibiotics within 3 days after the onset of the symptoms, but this is often impractical [5, 30]. According to the published guidelines of IDSA, ACG, AMSUS, AAP, and the International Society of Travel Medicine (ISTM), first-line antibiotics for intestinal Campylobacter infection are macrolides (especially azithromycin), given the low resistance rate, and fluoroquinolones are second-line agents [5, 17, 23-25]. The antibiotic therapy for Campylobacter enteritis usually lasts 3 days or until symptom resolution, but longer courses of 7 - 14 days should be considered in complicated cases or immunosuppressed patients [5, 17, 23, 24] (Table 3). Fluoroquinolones, tetracyclines, and trimethoprim-sulfamethoxazole (TMP-SMX) are alternative agents due to their higher resistance rate and unfavorable safety profile for pediatric patients [5, 11, 17, 23, 24]. B-lactams are generally considered ineffective, although recent epidemiologic data show minimal resistance to amoxicillin/clavulanate, and third-generation cephalosporins may also be effective [5, 11, 12, 16, 31]. Carbapenems are the most effective antibiotics; they should be administered with or without aminoglycosides in patients with severe campylobacteriosis and intolerance to oral agents [5]. Recently, increasing rates of antibiotic resistance have been reported globally due to the overuse of antibiotics in patients with infectious diarrhea and during meat production [5, 11, 20, 23, 36].
Campylobacter bloodstream infection is a rare manifestation which limits the available data on its management (Table 3 and Fig. 5). A study revealed that the empiric use of third-generation cephalosporins among patients with Campylobacter non-fetus bacteremia and the empiric use of fluoroquinolones among patients with Campylobacter fetus bacteremia are associated with increased mortality [12]. Thus, the role of antibiotics in managing Campylobacter bacteremia is controversial, but aminoglycosides and carbapenems are considered first-line options [5, 12, 16, 35] (Table 3). Carbapenems are preferred, but the combination of carbapenems and aminoglycosides, e.g., meropenem and gentamicin, should be considered in life-threatening infections [5, 35]. Although there are no official guidelines regarding the duration of the antibiotic treatment, it should be prolonged to prevent the recurrence of Campylobacter bacteremia. An antibiotic course of 7 - 14 days is a general rule for extra-intestinal infections, but a minimum of 3 - 4 weeks is recommended in bacteremic patients [5, 16]. More prolonged courses (weeks to years) with multiple antibiotics and adequate IVIG are necessary for patients with X-linked agammaglobulinemia [31]. Surgery may be required for the management of endovascular infection [16].
CNS involvement is common in patients with extraintestinal Campylobacter infection. The empiric antibiotic regimen (ampicillin plus gentamicin or ampicillin plus cefotaxime) for neonatal meningitis may provide adequate coverage of Campylobacter meningitis, as in our case [4, 22, 27]. However, the first-line antibiotics for Campylobacter meningitis are carbapenems and aminoglycosides; carbapenems are preferred over aminoglycosides due to higher penetration in the CNS and better safety [4, 5, 27] (Table 3). Meropenem is less likely to cause seizures than imipenem [37]. Generally, complicated Campylobacter infections are treated for 7 - 14 days as mentioned above, but antibiotic treatment in Campylobacter meningitis is recommended for at least 3 - 4 weeks, depending on the complications and the clinical course [4, 5, 16, 27]. Our neonate got clinically better with cefotaxime to which Campylobacter coli was sensitive hence we did not add meropenem. Parenteral administration of carbapenems and aminoglycosides provides adequate clearance of the extraintestinal Campylobacter infection. However, to prevent the recurrence or a nosocomial outbreak of Campylobacter infection, additional treatment with oral antibiotics, such as azithromycin or doxycycline, for GI decontamination has been proposed [4, 18, 30, 31] (Fig. 5). Additionally, de-escalation to oral amoxicillin with or without clavulanic acid after a successful trial of carbapenem has been proposed [16] (Fig. 5). In our case, azithromycin was given for 5 days for sterilization of the GI tract which could be the possible yet not confirmed route of infection.
Learning points
Campylobacter coli is a rare cause of late-onset neonatal meningitis, but it should be suspected based on dietary or family history. This case report highlights the importance of considering Campylobacter spp as one of the bacterial causes of neonatal sepsis and meningitis, especially when the initial cultures are negative. The clinical presentation of Campylobacter infection ranges from asymptomatic carriage to severe systemic disease. Severe complications, such as bacteremia and meningitis, are uncommon (less than 1% of cases). The isolation of Campylobacter spp in blood or CSF cultures without microaerophilic conditions is demanding. Stool cultures remain the gold-standard diagnostic method of campylobacteriosis, but the contribution of newer techniques, such as NAAT and ICT, is essential. Although Campylobacter enteritis is either self-limited or treated with macrolides, it is recommended to treat invasive Campylobacter infections with parenteral carbapenems to prevent relapse. However, first-line antibiotics for neonatal sepsis, such as third-generation cephalosporins, may effectively spare carbapenems based on antibiotic susceptibility testing. A 7 to 14-day antibiotic course is needed to treat invasive Campylobacter infections, and especially in Campylobacter meningitis cases, antibiotic treatment is recommended for at least 3 - 4 weeks depending on the complications and the clinical course. There are limited cases of Campylobacter neonatal invasive infections in literature, and thus further research is needed to establish guidelines for the earlier diagnosis and more effective treatment of these infections.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Written informed consent was obtained from the patient’s parents.
Author Contributions
Conceptualization: DK, CC, and DG. Data curation: DK and FK. Investigation: DK and CC. Methodology: DK, CC, and DG. Project administration: DG. Supervision: DG and PG. Validation: FK, GD, SF, PE, LL, DA, PG, and DG. Visualization: CC and DS. Writing - original draft: DK and CC. Writing - review editing: DK, CC, EK, FK, GD, SF, PE, LL, DA, DS, PG, and DG.
Date Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
AAP: American Academy of Pediatrics; ACG: American College of Gastroenterology; AMSUS: Association of the Military Surgeons in the United States; CIDT: culture-independent technique; CNS: central nervous system; CRP: C-reactive protein; CSF: cerebrospinal fluid; GBS: group B Streptococcus; GI: gastrointestinal; HIV: human immunodeficiency virus; ICT: immunochromatography; IDSA: Infectious Diseases Society of America; IgA: immunoglobulin A; IgG: immunoglobulin G; IgM: immunoglobulin M; ISTM: International Society of Travel Medicine; IVIG: intravenous immunoglobulin; LYMPH: lymphocytes; NAAT: nucleic acid amplification test; NEUT: neutrophils; PCR: polymerase chain reaction; PID: pelvic inflammatory disease; TMP-SMX: trimethoprim-sulfamethoxazole; VBNCs: viable but non-culturable cells; WBCs: white blood cells
| References | ▴Top |
- Thomas R, Bijlsma MW, Goncalves BP, Nakwa FL, Velaphi S, Heath PT. Long-term impact of serious neonatal bacterial infections on neurodevelopment. Clin Microbiol Infect. 2024;30(1):28-37.
doi pubmed - Edwards MS, Baker CJ. Bacterial meningitis in the neonate: clinical features and diagnosis. UpToDate. 2024.
- Trujillo-Gomez J, Navarro CE, Atehortua-Munoz S, Florez ID. Acute infections of the central nervous system in children and adults: diagnosis and management. Minerva Med. 2024;115(4):476-502.
doi pubmed - Botelho T, Peixoto D, Campos P, Alferes AP, Almiro MM, Flores MM. Campylobacter jejuni invasive infection in a 1-month-old infant. J Paediatr Child Health. 2022;58(11):2116-2117.
doi pubmed - Same RG, Tamma PD. Campylobacter infections in children. Pediatr Rev. 2018;39(11):533-541.
doi pubmed - Liu YH, Yamazaki W, Huang YT, Liao CH, Sheng WH, Hsueh PR. Clinical and microbiological characteristics of patients with bacteremia caused by Campylobacter species with an emphasis on the subspecies of C. fetus. J Microbiol Immunol Infect. 2019;52(1):122-131.
doi pubmed - Hsieh YH, Simpson S, Kerdahi K, Sulaiman IM. A comparative evaluation study of growth conditions for culturing the isolates of Campylobacter spp. Curr Microbiol. 2018;75(1):71-78.
doi pubmed - Bessede E, Asselineau J, Perez P, Valdenaire G, Richer O, Lehours P, Megraud F. Evaluation of the diagnostic accuracy of two immunochromatographic tests detecting campylobacter in stools and their role in campylobacter infection diagnosis. J Clin Microbiol. 2018;56(4):e01567-17.
doi pubmed - Harvala H, Ydring E, Brytting M, Soderblom T, Makitalo B, Wallensten A, Wisell KT, et al. Increased number of Campylobacter bacteraemia cases in Sweden, 2014. Clin Microbiol Infect. 2016;22(4):391-393.
doi pubmed - Karofylakis E, Gkrania-Klotsas E, Uttenthal B, Kumararatne D. Resolution of hypogammaglobulinemia-associated recurrent Campylobacter bacteraemia after hematopoietic cell transplantation (HCT). J Allergy Clin Immunol Glob. 2025;4(1):100378.
doi pubmed - Hussein K, Raz-Pasteur A, Shachor-Meyouhas Y, Geffen Y, Oren I, Paul M, Kassis I. Campylobacter bacteraemia: 16 years of experience in a single centre. Infect Dis (Lond). 2016;48(11-12):796-799.
doi pubmed - Pacanowski J, Lalande V, Lacombe K, Boudraa C, Lesprit P, Legrand P, Trystram D, et al. Campylobacter bacteremia: clinical features and factors associated with fatal outcome. Clin Infect Dis. 2008;47(6):790-796.
doi pubmed - Francois R, Yori PP, Rouhani S, Siguas Salas M, Paredes Olortegui M, Rengifo Trigoso D, Pisanic N, et al. The other Campylobacters: Not innocent bystanders in endemic diarrhea and dysentery in children in low-income settings. PLoS Negl Trop Dis. 2018;12(2):e0006200.
doi pubmed - Louwen R, van Baarlen P, van Vliet AH, van Belkum A, Hays JP, Endtz HP. Campylobacter bacteremia: a rare and under-reported event? Eur J Microbiol Immunol (Bp). 2012;2(1):76-87.
doi pubmed - Baek YJ, Song JE, Kim EJ, Choi H, Sohn Y, Jeon YD, Lee EH, et al. Trends, clinical characteristics, antimicrobial susceptibility patterns, and outcomes of Campylobacter bacteraemia: a multicentre retrospective study. Infection. 2024;52(3):857-864.
doi pubmed - Gazaigne L, Legrand P, Renaud B, Bourra B, Taillandier E, Brun-Buisson C, Lesprit P. Campylobacter fetus bloodstream infection: risk factors and clinical features. Eur J Clin Microbiol Infect Dis. 2008;27(3):185-189.
doi pubmed - Shane AL, Mody RK, Crump JA, Tarr PI, Steiner TS, Kotloff K, Langley JM, et al. 2017 infectious diseases society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis. 2017;65(12):e45-e80.
doi pubmed - Morooka T, Takeo H, Yasumoto S, Mimatsu T, Yukitake K, Oda T. Nosocomial meningitis due to Campylobacter fetus subspecies fetus in a neonatal intensive care unit. Acta Paediatr Jpn. 1992;34(5):530-533.
doi pubmed - Lee MM, Welliver RC, La Scolea LJ, Jr. Campylobacter meningitis in childhood. Pediatr Infect Dis. 1985;4(5):544-547.
doi pubmed - Gribble MJ, Salit IE, Isaac-Renton J, Chow AW. Campylobacter infections in pregnancy. Case report and literature review. Am J Obstet Gynecol. 1981;140(4):423-426.
doi pubmed - Rettig PJ. Campylobacter infections in human beings. J Pediatr. 1979;94(6):855-864.
doi pubmed - Goossens H, Henocque G, Kremp L, Rocque J, Boury R, Alanio G, Vlaes L, et al. Nosocomial outbreak of Campylobacter jejuni meningitis in newborn infants. Lancet. 1986;2(8499):146-149.
doi pubmed - Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602-622.
doi pubmed - Riddle MS, Martin GJ, Murray CK, Burgess TH, Connor P, Mancuso JD, Schnaubelt ER, et al. Management of acute diarrheal illness during deployment: a deployment health guideline and expert panel report. Mil Med. 2017;182(S2):34-52.
doi pubmed - Taylor DN, Hamer DH, Shlim DR. Medications for the prevention and treatment of travellers' diarrhea. J Travel Med. 2017;24(suppl_1):S17-S22.
doi pubmed - Valencak-Ignjatic I, Krajcar N, Didovic D, Roglic S, Butic I, Jelic M, Jednacak H, et al. Campylobacter jejuni subdural hygroma infection in a 2-year old boy: case report and a brief literature review. BMC Infect Dis. 2022;22(1):700.
doi pubmed - Kusulja M, Santini M, Margetic K, Guzvinec M, Soprek S, Butic I, Tambic Andrasevic A. Meningitis caused by Campylobacter jejuni: a case presentation and literature review. Acta Clin Belg. 2021;76(4):318-323.
doi pubmed - Arakawa Y, Yagi Y, Mimoto A, Nishida Y, Kuwana S, Nakai E, Ueba T, et al. Management of a large cerebral abscess in children caused by campylobacter gracilis: a case report and review of the literature. Cureus. 2024;16(6):e62744.
doi pubmed - Amoah KKA, Beach IR, Teague JM, Olszewski AM, DeWitt JC, Ducis KA. Campylobacter fetus seeding of a cavernous malformation resulting in brain abscess: case report and literature review. Childs Nerv Syst. 2023;39(12):3627-3631.
doi pubmed - Ternhag A, Asikainen T, Giesecke J, Ekdahl K. A meta-analysis on the effects of antibiotic treatment on duration of symptoms caused by infection with Campylobacter species. Clin Infect Dis. 2007;44(5):696-700.
doi pubmed - Zhuo R, Younes RL, Ward K, Yang S. Carbapenem resistant Campylobacter jejuni bacteremia in a Bruton's X-linked agammaglobulinemia patient. Eur J Clin Microbiol Infect Dis. 2024;43(12):2459-2463.
doi pubmed - Khairullah AR, Yanestria SM, Effendi MH, Moses IB, Jati Kusala MK, Fauzia KA, Ayuti SR, et al. Campylobacteriosis: A rising threat in foodborne illnesses. Open Vet J. 2024;14(8):1733-1750.
doi pubmed - Njoga EO, Nnaemeka VC, Jaja IF, Oguttu JW, Nwanta JA, Chah KF. Systematic review and meta-analysis of Campylobacter species infections in humans and food-producing animals in Nigeria, 2002-2023: The imperative of a One Health control approach. One Health. 2025;20:101029.
doi pubmed - Graham A, Hawkins L, Balasegaram S, Narasimhan S, Wain J, Clarke J, Manuel R. A decade of Campylobacter and Campylobacter bacteraemias in a district general hospital and the surrounding London and South East region, England. J Infect. 2024;88(1):15-20.
doi pubmed - Alnimr AM. A case of bacteremia caused by Campylobacter fetus: an unusual presentation in an infant. Infect Drug Resist. 2014;7:37-40.
doi pubmed - Martora F, Pagliuca C, Della Pepa ME, Della Rocca MT, Curto S, Iovene MR, Vitiello M. Campylobacter jejuni bacteremia in Italian pediatric patients with acute lymphoblastic leukemia: Report of two cases. New Microbiol. 2020;43(2):96-98.
pubmed - Roger C, Louart B. Beta-lactams toxicity in the intensive care unit: an underestimated collateral damage? Microorganisms. 2021;9(7):1505.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.