| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com |
Case Report
Volume 16, Number 6, June 2025, pages 195-200
Successful Treatment of Primary Central Nervous System T-Cell Lymphoma With Induction Chemotherapy Followed by Consolidation With High-Dose Chemotherapy and Autologous Stem Cell Rescue
Nolan Holleya, d, Shanawar Warisa, Huda Elzahranyb, David Howellb, Cara Randallb, Adnan Saifuddina, Salah Ud Din Safic
aDepartment of Medicine, West Virginia University, Morgantown, WV 26506, USA
bDepartment of Pathology, Anatomy and Laboratory Medicine, West Virginia University, Morgantown, WV 26506, USA
cDepartment of Medical Oncology, West Virginia University Cancer Institute, Morgantown, WV 26506, USA
dCorresponding Author: Nolan R. Holley, Department of Medicine, West Virginia University, Morgantown, WV 26506, USA
Manuscript submitted April 14, 2025, accepted May 21, 2025, published online June 9, 2025
Short title: HDC-ASCT for PCNSTL
doi: https://doi.org/10.14740/jmc5130
| Abstract | ▴Top |
Primary central nervous system T-cell lymphoma (PCNSTL) is an exceptionally rare subtype of non-Hodgkin lymphoma, comprising only 2% of primary CNS lymphoma cases. Due to its rarity, PCNSTL is often misdiagnosed, lacks standardized treatment guidelines, and carries a poor prognosis. We present a unique case of a 59-year-old-woman with a history of hypertension and hyperlipidemia who initially presented with sudden-onset aphasia and right-sided weakness. Suspected initially of having a cerebrovascular accident (CVA), she received tenecteplase and underwent investigation of CVA, which was largely unremarkable. Six months later, she returned with progressive nausea, vomiting, confusion, and word-finding difficulties. A magnetic resonance imaging (MRI) of the brain showed lesions in the left cerebellum and frontal lobe with vasogenic edema. A suboccipital craniotomy and biopsy confirmed anaplastic lymphoma kinase (ALK)-negative anaplastic large cell lymphoma. Positron emission tomography-computed tomography (PET-CT) showed no systemic disease and cerebrospinal fluid (CSF) analysis showed lymphomatous involvement. The patient was initiated on six cycles of methotrexate, cytarabine, and thiotepa (MATRix regimen without rituximab), followed by high-dose chemotherapy (HDC) with carmustine and thiotepa and autologous stem cell transplantation (ASCT). She tolerated treatment and transplant without complications and remains in complete remission 18 months post-transplant. To our knowledge, this is the first reported case of PCNSTL treated successfully with MATRix followed by HDC-ASCT. This case highlights the importance of considering rare CNS lymphomas in patients with atypical neurologic presentations and suggests the use of HDC-ASCT as a promising approach in a disease with no established standard of care.
Keywords: Primary CNS T-cell lymphoma; Autologous stem cell transplant; Complete remission; High-dose chemotherapy
| Introduction | ▴Top |
Primary central nervous system lymphoma (PCNSL) is an uncommon malignancy that accounts for less than 10% of CNS malignancies and less than 5% of non-Hodgkin lymphomas [1]. PCNSL is most commonly of B-cell lineage. Primary CNS T-cell lymphoma (PCNSTL) only accounts for 2% of all PCNSLs. Because of its rarity, clinicians face an ongoing diagnostic and therapeutic challenge [2]. Patients typically present with non-specific neurological symptoms including but not limited to focal neurological deficits, personality changes, signs and symptoms of increased intracranial pressure, and visual disturbances [3]. Patients with such presentations are commonly subjected to neurological imaging, lumbar puncture with cerebrospinal fluid (CSF) analysis, surgical resection with biopsy, and immunohistochemical and molecular analysis of resected tissue for elucidation of an accurate diagnosis [1, 2].
Pathological analysis of such specimens can present difficulty. Most cases are ultimately classified as peripheral T-cell lymphoma, not otherwise specified (PTCL, NOS). Some cases are identified as anaplastic large cell lymphoma (ALCL) that is further classified based on anaplastic lymphoma kinase (ALK) expression. Unsurprisingly, specimens are often CD3 and CD8-positive. However, they have also been shown to frequently express cytotoxic markers such as TIA1 and granzyme-B as well as mutations in DNMT3A, KRAS, JAK3, STAT3, STAT5B, GNB1, and TET2 [2].
High-dose methotrexate (HD-MTX) remains the standard-of-care backbone in treatment of PCNSL. HD-MTX is often used in tandem with temozolomide or cytarabine for initial therapy. Following HD-MTX induction therapy, many patients undergo consolidation therapy. Consolidation with high-dose chemotherapy and autologous stem cell transplantation (HDC-ASCT) is being increasingly considered [1, 3]. This strategy has been shown to improve survival outcomes [4], with immense benefit for fit patients with relapsed and refractory PCNSL [5]. However, these findings are in patients with primary CNS B-cell lymphoma (PCNSBL). To date, there are no recorded cases of HDC-ASCT consolidation for the treatment of PCNSTL. This case illustrates the successful use of HDC-ASCT in a patient with PCNSTL, which is a treatment approach not previously documented in the literature.
| Case Report | ▴Top |
A 59-year-old female with a pertinent past medical history of hypertension and hyperlipidemia presented to her local emergency department with aphasia and weakness. These symptoms were assessed to be concerning for cerebrovascular accident (CVA) for which tissue plasminogen activator (tPA) was administered. The patient was then transferred to our academic medical center for tertiary stroke evaluation. Standard CVA investigations including magnetic resonance imaging (MRI) of the brain, imaging of the intracranial and extracranial vessels, and echocardiography were largely unremarkable; MRI showed no concerns for infarct, hemorrhage, or malignancy. Thus, the patient was discharged home without further intervention.
Four months later, she developed progressively worsening nausea, vomiting, confusion, and word-finding difficulties over the course of 2 months, which led to repeat presentation to our facility 6 months following prior discharge. MRI of the brain at this time revealed lesions in the left cerebellar and left frontal lobe regions with surrounding vasogenic edema (Fig. 1). Given these findings, she underwent left suboccipital craniotomy with open biopsy of the lesion. Histologic examination of the biopsy specimen revealed an abnormal infiltrate of large, pleomorphic cells with irregular nuclear contours, prominent nucleoli, and moderate amounts of cytoplasm. Occasional cells have features of “hallmark” cells, with deeply indented, eccentric kidney or horseshoe-shaped nuclei. Mitotic figures and tumor necrosis were readily identifiable (see Fig. 2 for histologic findings). By immunohistochemical (IHC) analysis, the neoplastic cells were strongly positive for CD3 and CD30 and were negative for ALK1, CD15, CD20, and PAX5 (see Fig. 3 and Table 1 for additional IHC findings). A diagnosis of aggressive T-cell lymphoma most consistent with ALK-negative ALCL was rendered. T-cell clonality studies performed by polymerase chain reaction (PCR) analysis showed clonal T-cell receptor beta (TCRB) and T-cell receptor gamma (TCRG) rearrangements. Fluorescence in situ hybridization (FISH) studies were negative for ALK, DUSP22, and TP63 rearrangements. A 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) scan showed localized intracranial disease and a bone marrow biopsy showed normocellular marrow with no evidence of lymphomatous involvement. CSF analysis revealed markedly elevated protein (735 mg/dL; reference range 14 - 45 mg/dL), low glucose (3 mg/dL; reference range 40 - 75 mg/dL), and high nucleated cell count (80 nucleated cells/µL; reference range 0 - 5 nucleated cells/µL). Cytologic examination revealed numerous highly atypical lymphocytes indicative of lymphomatous involvement, for which six doses of intrathecal methotrexate (12 mg each) were administered.
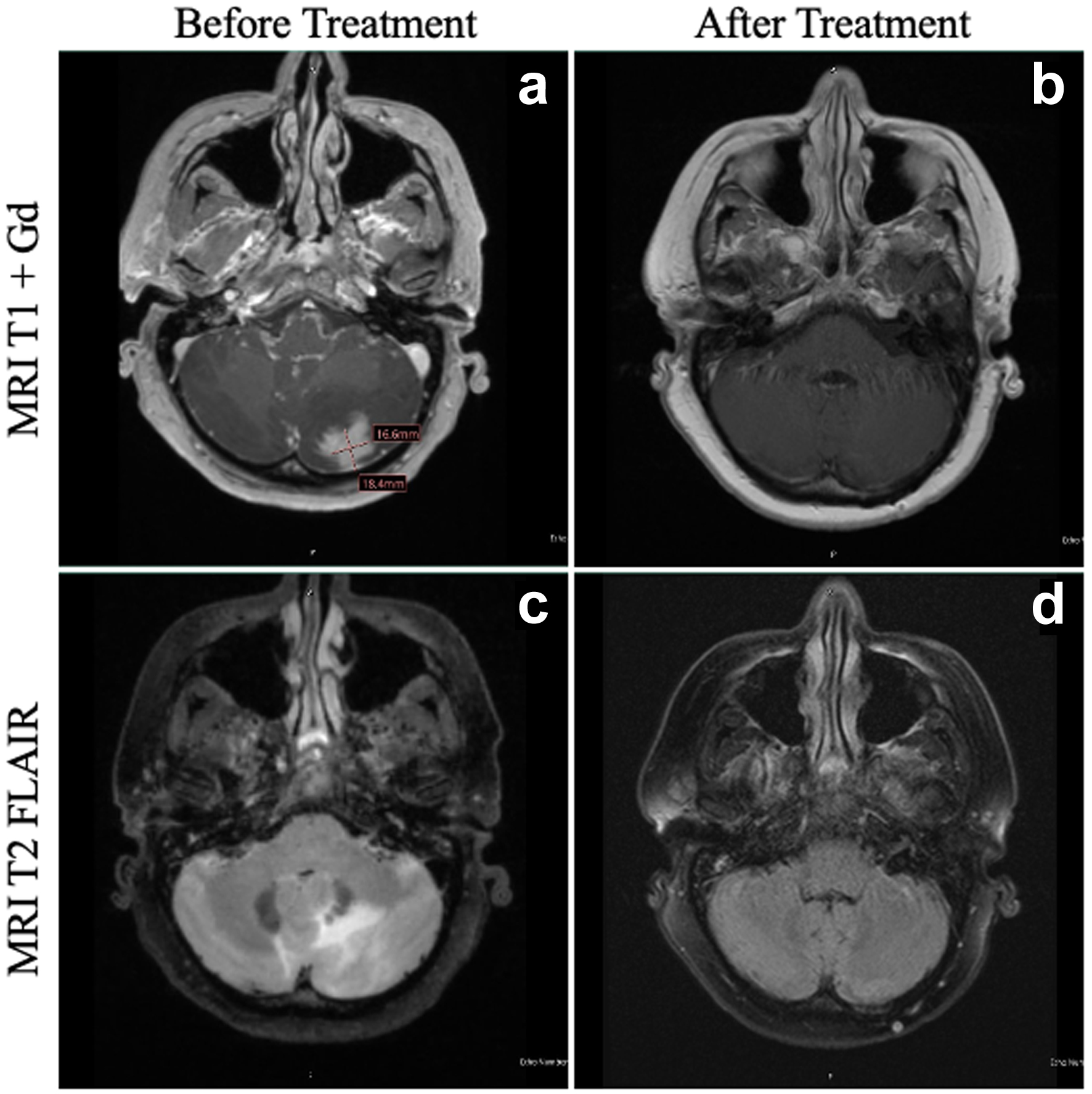 Click for large image | Figure 1. MRI of the patient’s brain before and after HDC-ASCT. At the time of diagnosis of PCNSTL, the patient had a lesion in the left cerebellum on T1 + Gd (a), with significant vasogenic edema evident on T2 FLAIR (c). At 1 year following HDC-ASCT, the patient has complete resolution of both the area of the primary cerebellar lesion (b) as well as total resolution of vasogenic edema (d). FLAIR: fluid-attenuated inversion recovery; HDC-ASCT: high-dose chemotherapy followed by autologous stem cell transplantation; MRI: magnetic resonance imaging; PCNSTL: primary CNS T-cell lymphoma. |
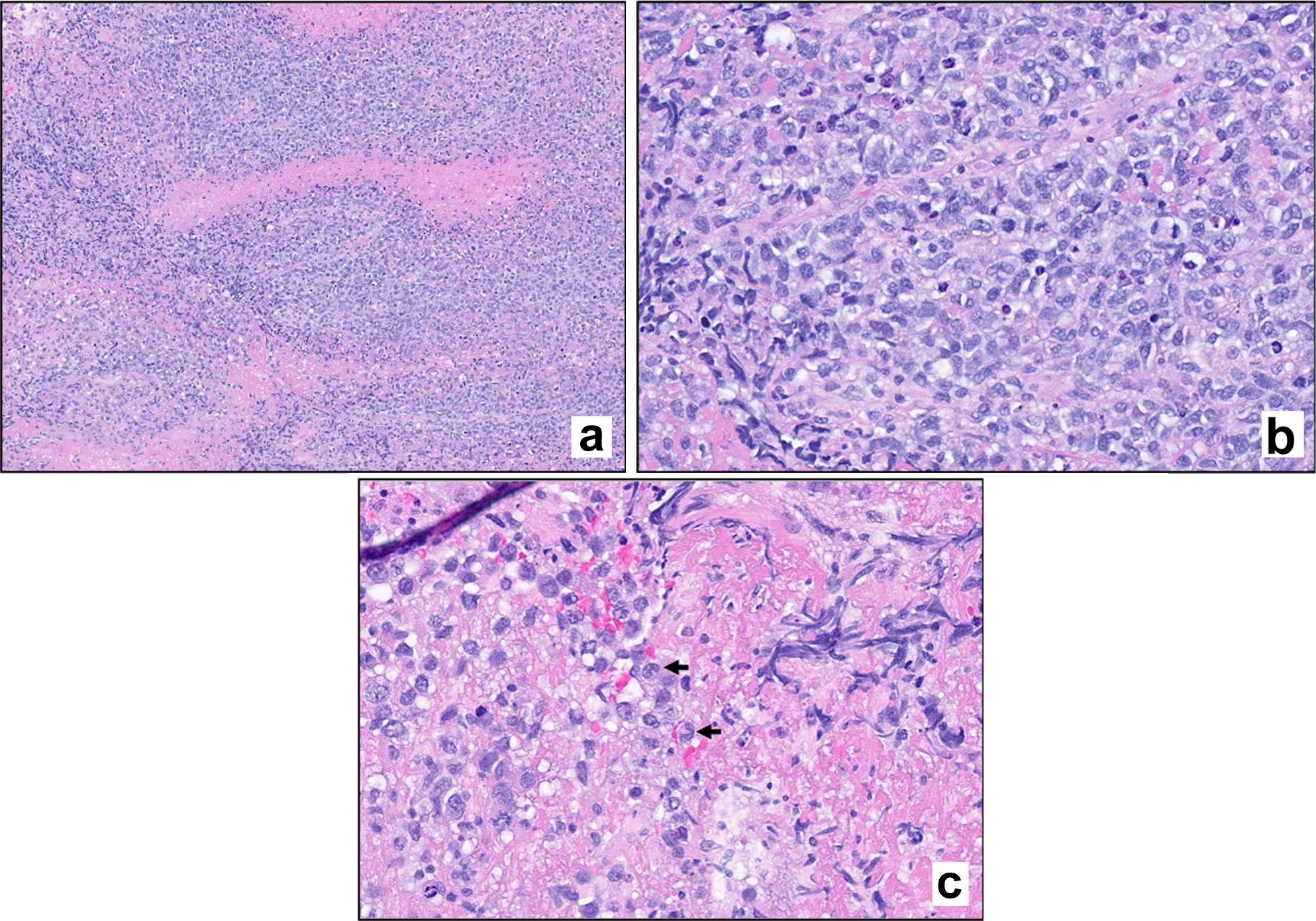 Click for large image | Figure 2. Hematoxylin and eosin (H&E) stain. Stained sections reveal fragments of brain parenchyma with a diffuse infiltrate of large atypical lymphoid cells. Mitotic figures are evident (a, × 100 magnification; b, × 400 magnification). Scattered cells exhibit morphologic features consistent with hallmark cells (c, arrows). |
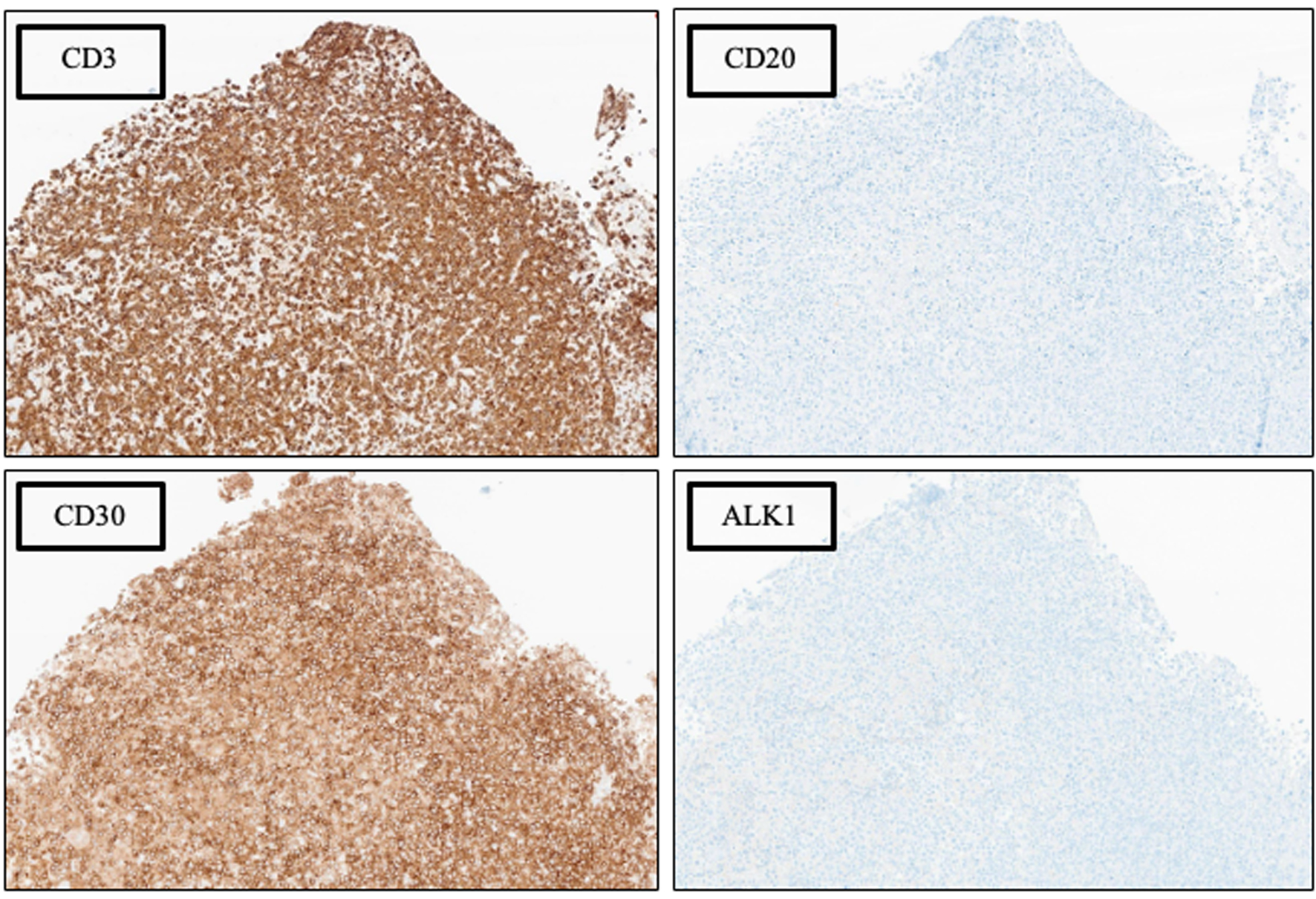 Click for large image | Figure 3. Phenotypic characterization by immunohistochemical (IHC) analysis, × 100 magnification. See Table 1 for additional IHC findings. |
 Click to view | Table 1. Additional Immunohistochemical and in situ Hybridization Results |
Following initial diagnosis, investigation, and staging, the patient was initiated on induction chemotherapy consisting of six cycles of methotrexate (3.5 g/m2), thiotepa (30 mg/m2), and cytarabine (2 g/m2) (“MATRix” regimen without rituximab) [6]. Induction chemotherapy was followed by consolidation with HDC consisting of thiotepa (5 mg/kg) and carmustine (400 mg/m2) (TT-BCNU). She then underwent an ensuing ASCT rescue. The patient tolerated the entirety of her treatment well, with no dose reductions, adverse events, or complications. Approximately 18 months following HDC-ASCT, the patient remains in complete remission, with no evidence of disease on her most recent brain MRI (Fig. 1). She is asymptomatic and has a normal functional status.
| Discussion | ▴Top |
We present the first case of a patient with PCNSTL who underwent HDC-ASCT consolidation and now in complete remission (Fig. 4). In general, PCNSL is associated with a poor prognosis. The 5-year survival rate for PCNSL is only 30% [7]. This statistic is most representative of a patient population with PCNSL of B-cell lineage, given the aforementioned prevalence of this phenotype. It is reasonable to assume that the 5-year survival rate for PCNSTL falls below this value given the rarity of disease and lack of clear guidance on its management. While the 5-year survival data are grim, this disease is made further dismal given that early mortality within 60 days of diagnosis occurs in approximately 9.8% of patients with PCNSL [8].
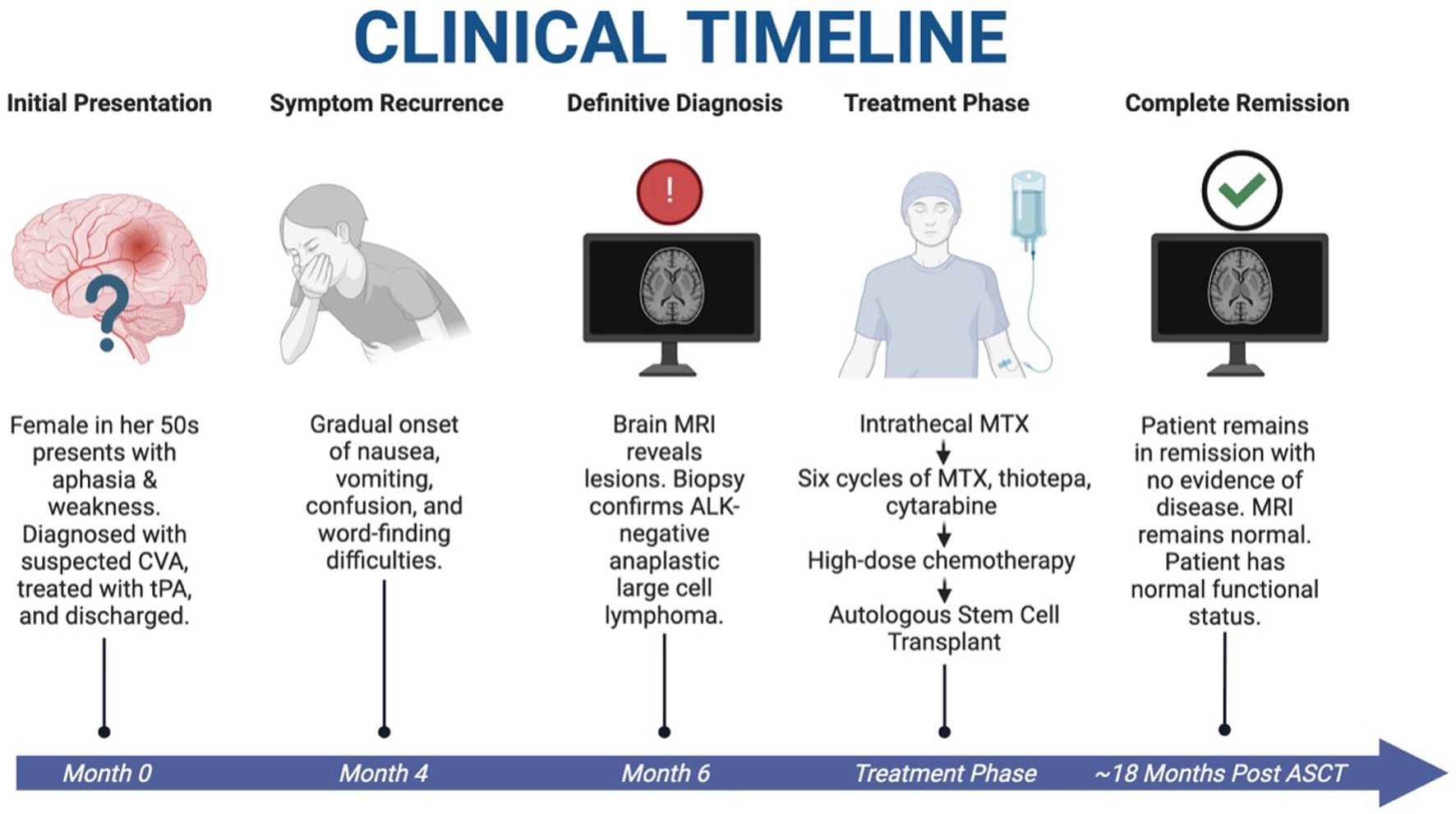 Click for large image | Figure 4. Clinical timeline of case. A patient with initial stroke-like symptoms was found to have PCNSTL. She underwent HDC-ASCT and is currently in complete remission with resolution of symptoms and restoration of normal functional status at approximately 18 months after HDC-ASCT. HDC-ASCT: high-dose chemotherapy followed by autologous stem cell transplantation; PCNSTL: primary CNS T-cell lymphoma. Created in BioRender. Holley, N. (2025) https://BioRender.com/kdfox90. |
Many promising advancements are being made in the pursuit of improving outcomes for patients with PCNSL. HD-MTX with either temozolomide or cytarabine remains a mainstay in the upfront management of PCNSL. After assessing the initial response to induction chemotherapy, patients often receive consolidation treatment. The consolidation can include whole-brain radiation therapy (WBRT), HDC-ASCT, or maintenance low-dose chemotherapy. The choice of consolidation is based on patient’s eligibility for transplant. WBRT is associated with significant morbidity in the form of neurotoxicity and is usually reserved for patients who are transplant ineligible [3]. HDC-ASCT was initially used with the aim of improved CNS chemotherapy bioavailability and to serve as a replacement of WBRT to reduce neurotoxicity [3]. This method was shown to be associated with improvement in complete remission and 2-year overall survival in relapsed and refractory PCNSL in early studies [9].
The blood-brain barrier acts as a sanctuary site for PCNSL. HDC allows for greater delivery of cytotoxic chemotherapy beyond the blood-brain barrier, allowing for significant therapeutic drug concentrations to be present at the site of malignancy. This may be especially beneficial for elimination of residual chemoresistant PCNSL cells present following initial treatment [10]. This pathophysiology translates into the clinical setting. In a recent phase 3 study evaluating patients with PCNSL receiving four cycles of rituximab, methotrexate, cytarabine, and thiotepa (MATRix) followed by HDC/ASCT, it was found that this population had significantly improved progression-free survival and 3-year overall survival compared to patients receiving conventional chemoimmunotherapy [6].
Despite these promising findings for HDC-ASCT for use in PCNSL, there remains no documented cases of its use specifically in PCNSTL. Furthermore, there remains no specific standard treatment protocol for PCNSTL, specifically. Thus, treatment of PCNSTL has followed a similar structure as the more common PCNSBL. However, these diseases have both pathophysiological and clinical differences. Firstly, rituximab therapy, which is a cornerstone of management for PCNSBL, has no utility in T-cell lymphomas, as these malignant cells should not express CD20. Clinically, its presentation represents an enigma given the lack of compelling research data yielding distinct and straightforward guidance [11]. Through this case report, we aim to contribute to the limited research in this area.
In this case, our patient presented at a relatively favorable age with good functional status. Our strategy to use HDC-ASCT consolidation was based on evidence available from PCNSBL management. Given that the patient was able to tolerate this treatment strategy, and at this time remains in complete remission, we suggest that in a young and fit patient with PCNSTL, HDC-ASCT is a feasible treatment modality. Many more cases and prospective studies are needed to make further substantial claims regarding the effectiveness of this intervention for patients with PCNSTL.
Conclusion
In summary, we present the case of a 59-year-old female with PCNSTL who remains in complete remission approximately 18 months after HDC-ASCT (Fig. 4). PCNSL, in general, is associated with a poor prognosis, highlighting the need for continued research to improve outcomes for these patients. PCNSTL, a rare subset of this disease, currently lacks clear management guidelines. While HDC-ASCT has been shown to improve event-free survival and overall survival in PCNSBL, it has not been previously used for PCNSTL. This report provides evidence supporting the feasibility of this regimen for patients with PCNSTL.
Learning points
PCNSTL is an exceptionally rare and aggressive malignancy that can present with nonspecific neurologic symptoms and may easily be misdiagnosed as more common conditions such as a CVA.
There is no established standard-of-care treatment for PCNTL, and management is often extrapolated from protocols used for management of PCNSBL.
This case demonstrates the feasibility and tolerability of MATRix chemotherapy followed by HDC-ASCT rescue in a patient with PCNSTL, resulting in durable complete remission.
HDC and stem cell transplantation may offer a promising management strategy for fit patients with PCNSTL, warranting further investigation in prospective studies.
Acknowledgments
The authors of this case report would like to acknowledge the patient for their willingness to contribute to medical literature.
Financial Disclosure
The authors of this case report have no financial disclosure or relevant funding sources.
Conflict of Interest
The authors of this case report have no conflict of interest.
Informed Consent
The authors of this case report have obtained informed consent.
Author Contributions
Dr. Nolan Holley: conceptualization, data curation, formal analysis, investigation, methodology, resources, visualization, and writing - original draft. Dr. Shanawar Waris: writing - original draft, writing - review and editing, and resources. Dr. Huda Elzahrany: investigation, formal analysis, data curation, visualization, and writing - original draft. Dr. Cara Randall: writing - review and editing, visualization, and supervision. Dr. David Howell: validation, supervision, and resources. Dr. Adnan Saifuddin: writing - original draft, writing - review and editing, and resources. Dr. Salah Ud Din Safi: conceptualization, formal analysis, investigation, methodology, supervision, project administration, and writing - review and editing.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ALK: anaplastic lymphoma kinase; ASCT: autologous stem cell transplantation; BCNU: carmistine (1,3-bis(2-chloroethyl)-1-nitrosurea); CNS: central nervous system; CSF: cerebrospinal fluid; CVA: cerebrovascular accident; FDG: fluorodeoxyglucose; FLAIR: fluid-attenuated inversion recovery; FISH: fluorescence in situ hybridization; H&E: hematoxylin and eosin; HDC: high-dose chemotherapy; HD-MTX: high-dose methotrexate; HDC-ASCT: high-dose chemotherapy followed by autologous stem cell transplantation; IHC: immunohistochemistry; MRI: magnetic resonance imaging; MTX: methotrexate; NOS: not otherwise specified; PCNSL: primary central nervous system lymphoma; PCNSBL: primary CNS B-cell lymphoma; PCNSTL: primary CNS T-cell lymphoma; PET: positron emission tomography; PTCL: peripheral T-cell lymphoma; TCRB: T-cell receptor beta; TCRG: T-cell receptor gamma; TT-BCNU: thiotepa and carmustine; WBRT: whole-brain radiation therapy
| References | ▴Top |
- Menon MP, Nicolae A, Meeker H, Raffeld M, Xi L, Jegalian AG, Miller DC, et al. Primary CNS T-cell lymphomas: a clinical, morphologic, immunophenotypic, and molecular analysis. Am J Surg Pathol. 2015;39(12):1719-1729.
doi pubmed - Bird CE, Traylor JI, Thomas J, Caruso JP, Kafka B, Rosado F, Blackburn KM, et al. Primary peripheral T-cell central nervous system lymphoma. Surg Neurol Int. 2021;12:465.
doi pubmed - Ferreri AJ. How I treat primary CNS lymphoma. Blood. 2011;118(3):510-522.
doi pubmed - Narvel H, Vegivinti CTR, Vikash S, et al. Autologous stem cell transplantation (ASCT) improves survival outcome in primary central nervous system lymphoma (PCNSL) in a minority rich, underserved inner city population in the real-world setting. Blood. 2022;140(Supplement 1):12146-12147.
doi - Steffanoni S, Calimeri T, Marktel S, Nitti R, Foppoli M, Ferreri AJM. Diagnosis and treatment using autologous stem-cell transplantation in primary central nervous system lymphoma: a systematic review. Cancers (Basel). 2023;15(2):526.
doi pubmed - Illerhaus G, Ferreri AJM, Binder M, et al. Effects on survival of non-myeloablative chemoimmunotherapy compared to high-dose chemotherapy followed by autologous stem cell transplantation (HDC-ASCT) as consolidation therapy in patients with primary CNS lymphoma - results of an international randomized phase III trial (MATRix/IELSG43). Blood. 2022;140(Supplement 2):LBA-3.
doi - Shiels MS, Pfeiffer RM, Besson C, Clarke CA, Morton LM, Nogueira L, Pawlish K, et al. Trends in primary central nervous system lymphoma incidence and survival in the U.S. Br J Haematol. 2016;174(3):417-424.
doi pubmed - Lin CH, Yang CF, Yang HC, Fay LY, Yeh CM, Kuan AS, Wang HY, et al. Risk prediction for early mortality in patients with newly diagnosed primary CNS lymphoma. J Cancer. 2019;10(17):3958-3966.
doi pubmed - Soussain C, Hoang-Xuan K, Taillandier L, Fourme E, Choquet S, Witz F, Casasnovas O, et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Societe Francaise de Greffe de Moelle Osseuse-Therapie Cellulaire. J Clin Oncol. 2008;26(15):2512-2518.
doi pubmed - Ferreri AJ, Illerhaus G. The role of autologous stem cell transplantation in primary central nervous system lymphoma. Blood. 2016;127(13):1642-1649.
doi pubmed - Chihara D, Oki Y. Central nervous system involvement in peripheral T cell lymphoma. Curr Hematol Malig Rep. 2018;13(1):1-6.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.