| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com |
Case Report
Volume 16, Number 5, May 2025, pages 181-186
High-Sensitivity Flow Cytometric Detection of a Small Circulating Population of Nodal T-Follicular Helper Cell Lymphoma Angioimmunoblastic Type Cells
Arianna Gattia, d , Silvia Franceschettib, Valentina Spezialea, Viviana Beatrice Vallib, Michela Draiscib, Cristina Campidellic, Bruno Brandoa, Irene Cupparia, Alessandro Corsob
aHematology Laboratory and Transfusion Center Department, ASST Ovest Milanese-Ospedale Legnano, 20025 Legnano (Milan), Italy
bHematology Department, ASST Ovest Milanese-Ospedale Legnano, 20025 Legnano (Milan), Italy
cAnatomical Pathology Department, ASST Ovest Milanese-Ospedale Legnano, 20025 Legnano (Milan), Italy
dCorresponding Author: Arianna Gatti, Hematology Laboratory and Transfusion Center, Legnano General Hospital, Legnano (Milan), Italy
Manuscript submitted February 6, 2025, accepted April 23, 2025, published online May 28, 2025
Short title: Detection of nTFHL by High-Sensitivity Flow Cytometry
doi: https://doi.org/10.14740/jmc5114
| Abstract | ▴Top |
Nodal T-follicular helper cell lymphoma angioimmunoblastic type (nTFHL-AI) is a rare and aggressive neoplasm of mature T-follicular helper cells. nTFHL-AI is characterized by polyclonal hypergammaglobulinemia, hemolytic anemia, circulating immune complexes, and cold agglutinins. nTFHL-AI is also often associated with B-cell or plasma cell expansion, mimicking B-cell lymphomas or plasma cell neoplasms. Therefore, the diagnosis of nTFHL-AI can sometimes be challenging and requires a specific immunophenotypic panel. However, the peripheral blood involvement in nTFHL-AI seems rare and has not been frequently addressed in the literature. We report the case of a 54-year-old man with multiple lymphadenopathies, hepatosplenomegaly, and skin rash, complaining of asthenia. Peripheral blood smear showed plasmacytoid cells and red cell rouleaux. A first flow cytometry screening panel of peripheral blood disclosed marked polyclonal plasmacytosis (12%). No mature B lymphocytes were detectable. In the suspicion of an nTFHL-AI, another flow cytometric panel was performed, including CD3, CD4, CD5, CD7, CD8, and CD10. The high-sensitivity flow cytometry analysis disclosed a small circulating population of atypical T cells (0.07%) expressing CD4+, CD3+, CD5+, CD10+, partially CD7+, and negative for CD8. Moreover, anti-TCRβ-chain constant region 1 (TRBC1) antibody (JOVI-1) was used to confirm the T-cell clonal restriction of this abnormal population. Immunohistochemistry on excised lymph node sections was carried out and confirmed the diagnosis of nTFHL-AI. In this case, the unexpected detection of a small circulating population of nTFHL-AI cells by high-sensitivity flow cytometry has prompted an extensive diagnostic workup leading rapidly to the correct diagnosis.
Keywords: Nodal T-follicular helper cell lymphoma angioimmunoblastic type; Angioimmunoblastic T-cell lymphoma; Peripheral blood involvement; Flow cytometry
| Introduction | ▴Top |
Nodal T-follicular helper cell lymphoma angioimmunoblastic type (nTFHL-AI), previously known as angioimmunoblastic T-cell lymphoma [1], is an aggressive neoplasm of mature T-follicular helper cells, accounting for 1-2% of all non-Hodgkin lymphomas. nTFHL-AI is characterized by some peculiar laboratory features, including polyclonal hypergammaglobulinemia, hemolytic anemia, circulating immune complexes, and cold agglutinins [2]. Skin lesions can range from urticaria to rashes or nodular tumors [3].
nTFHL-AI is also often associated with B-cell or plasma cell expansion, mimicking B-cell lymphomas or plasma cell neoplasms [4, 5]. Therefore, the diagnosis of nTFHL-AI can sometimes be challenging and requires a complete immunophenotypic and molecular workup. Bone marrow (BM) involvement is common and has been variably described in nearly 70% of cases [6]. However, the peripheral blood involvement in nTFHL-AI seems rare and has not been frequently addressed in the literature [7]. Multiparametric flow cytometry (MFC) analysis is described as an effective and useful method in the characterization of the leukemic phase of nTFHL-AI, rapidly enabling the correct diagnosis [8]. Rapid diagnosis and timely therapeutic measures are necessary due to the poor prognosis of nTFHL-AI patients, which entails a median survival of less than 3 years [9]. In this case report, we highlighted the role of high-sensitivity MFC analysis of peripheral blood that allowed the unexpected detection of a small circulating population of nTFHL-AI cells, leading rapidly to the correct diagnosis and allowing the start of intensive chemotherapy.
| Case Report | ▴Top |
We report the case of a 54-year-old man with multiple lymphadenopathies, hepatosplenomegaly, and skin rash, complaining of asthenia. Laboratory tests showed anemia grade 3 (Hb 67 g/L), lymphopenia (lymphocytes 2.7%, 0.3 × 109/L), increased level of lactate dehydrogenase (LDH, 495 U), preserved haptoglobin, and hypergammaglobulinemia. Direct Coombs test was positive for IgG and C3. Peripheral blood smear showed plasmacytoid cells and red cell rouleaux (Fig. 1). A first flow cytometry screening panel of peripheral blood, including CD19, CD20, CD38, CD45, CD56, CD138, and cytoplasmic light chains kappa and lambda, disclosed marked polyclonal plasmacytosis (12% of leucocytes), with virtual absence of mature B lymphocytes. The gating strategy was set to identify plasma cells with the co-expression of CD38 and CD138; the B-cell capture gate included CD19 and CD20. In the suspicion of an nTFHL-AI, another MFC panel was performed, including CD3, CD4, CD5, CD7, CD8, CD10, and CD45. CD45-positive cells were used as the denominator for calculations. The analysis disclosed a small circulating population of atypical T cells (0.07% out of 793,000 CD45+ white cells) expressing CD4+, CD3+, CD10+, with a bimodal expression of CD7+ (CD7 positive were 30%), strong positivity for CD5, and negativity for CD8 (Fig. 2). The expression of CD4 on abnormal cells was reduced if compared to the normal T CD4+ counterpart. Moreover, the anti-TCRβ-chain constant region 1 (TRBC1) antibody (JOVI-1), recently identified as a marker able to identify T-cell clonality, was used, confirming the T-cell clonal restriction of the abnormal nTFHL-AI population (Fig. 3). Immunohistochemistry (IHC) on excised lymph node sections was carried out. Small to medium-sized T CD4+ cells surrounding endothelial venules were observed. A proliferation of follicular dendritic cells was variably detected (Fig. 4a). Accompanying polymorphic inflammatory cells and CD8+ granzyme+ T cells were identified (Fig. 4b). The IHC analysis confirmed the presence of an atypical T-cell population expressing CD3+, CD2+, CD5+, and CD10+ with partial expression of CD7 and CD30 (Fig. 4c-f).
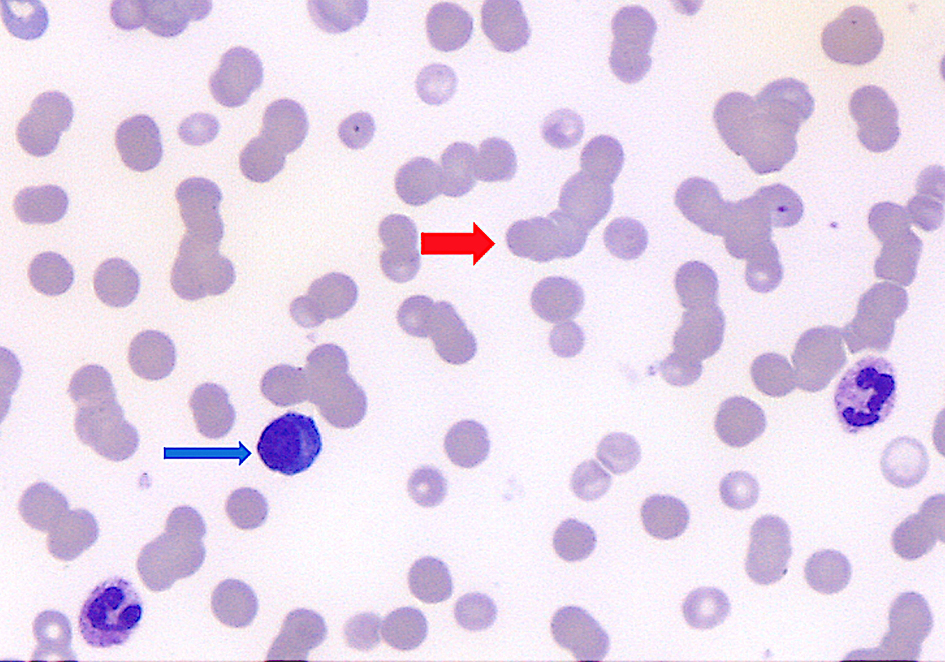 Click for large image | Figure 1. Peripheral blood smear showed plasmacytoid cells (blue arrow, approximately 5% of white cells) and red cell rouleaux (red arrow). |
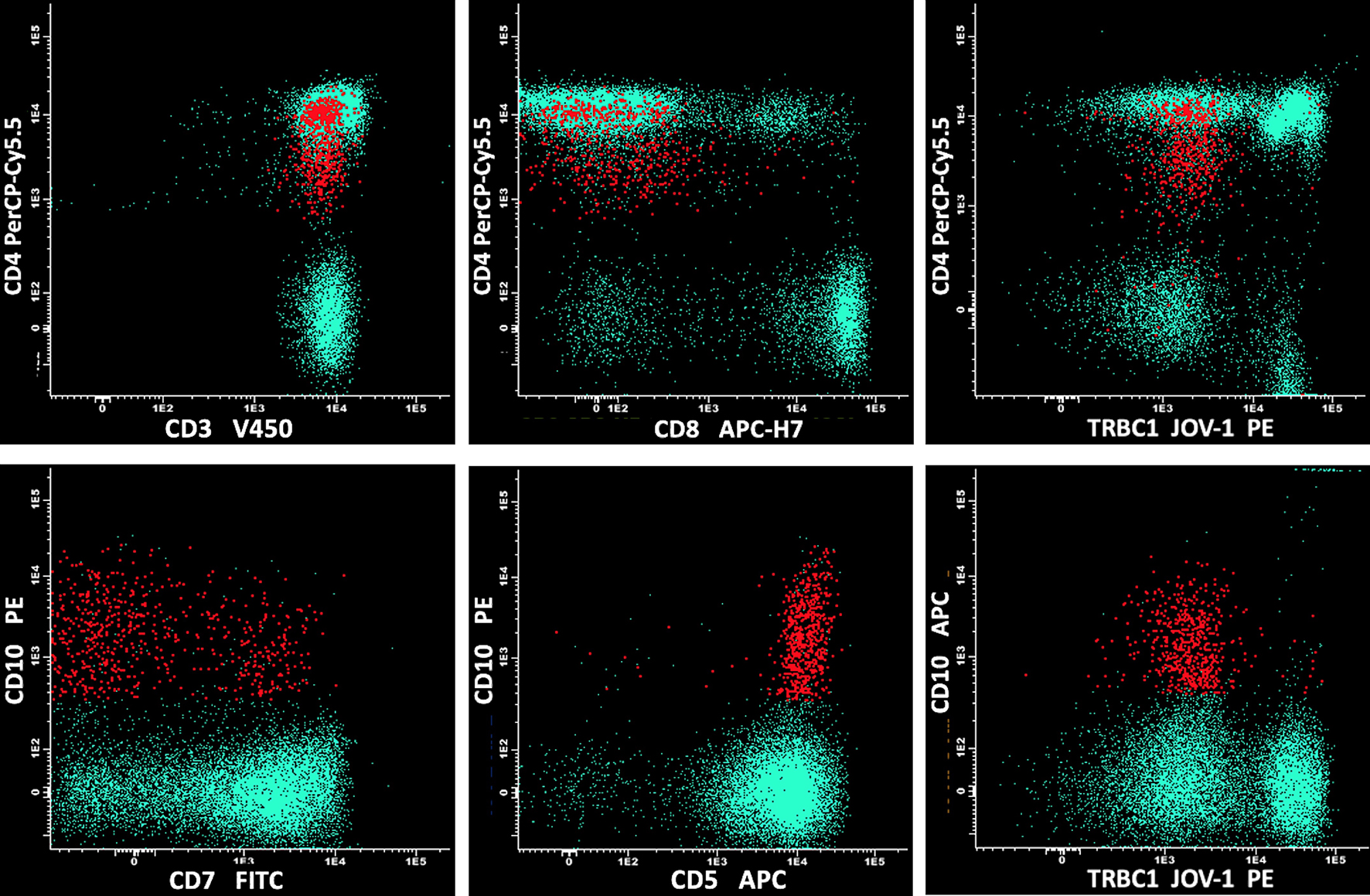 Click for large image | Figure 2. The MFC analysis included sequentially: CD45/side scatter, CD45+++/CD3+, and CD3+/CD10+, with further phenotypic characterization using CD4, CD5, CD7, and CD8. The analysis disclosed a small circulating population of atypical T cells (in red) expressing CD3+, CD10+, bimodal CD7+, and negativity for CD8, with strong positivity for CD5 and reduced expression of CD4, if compared to normal T CD4+ and CD8+ cell counterparts (in green). MFC: multiparametric flow cytometry. |
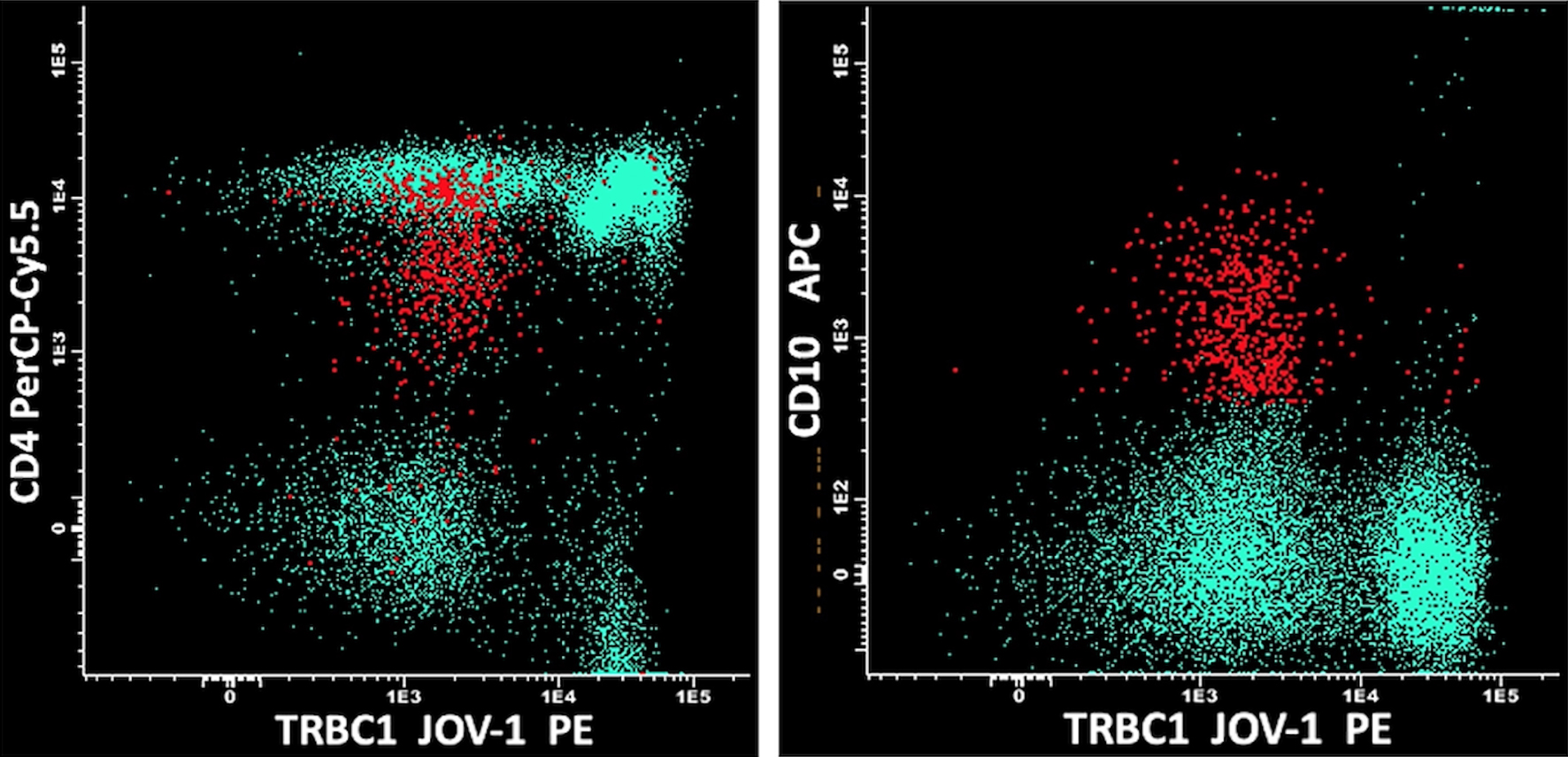 Click for large image | Figure 3. nTFHL-AI cells (in red) were negative for TRBC1 JOVI-1, confirming the T-cell clonal restriction. The normal T CD4+ and CD8+ cell counterpart (in green) showed bimodal expression of TRBC1 JOVI-1, indicating polyclonality. nTFHL-AI: nodal T-follicular helper cell lymphoma angioimmunoblastic type; TRBC1: TCRβ-chain constant region 1. |
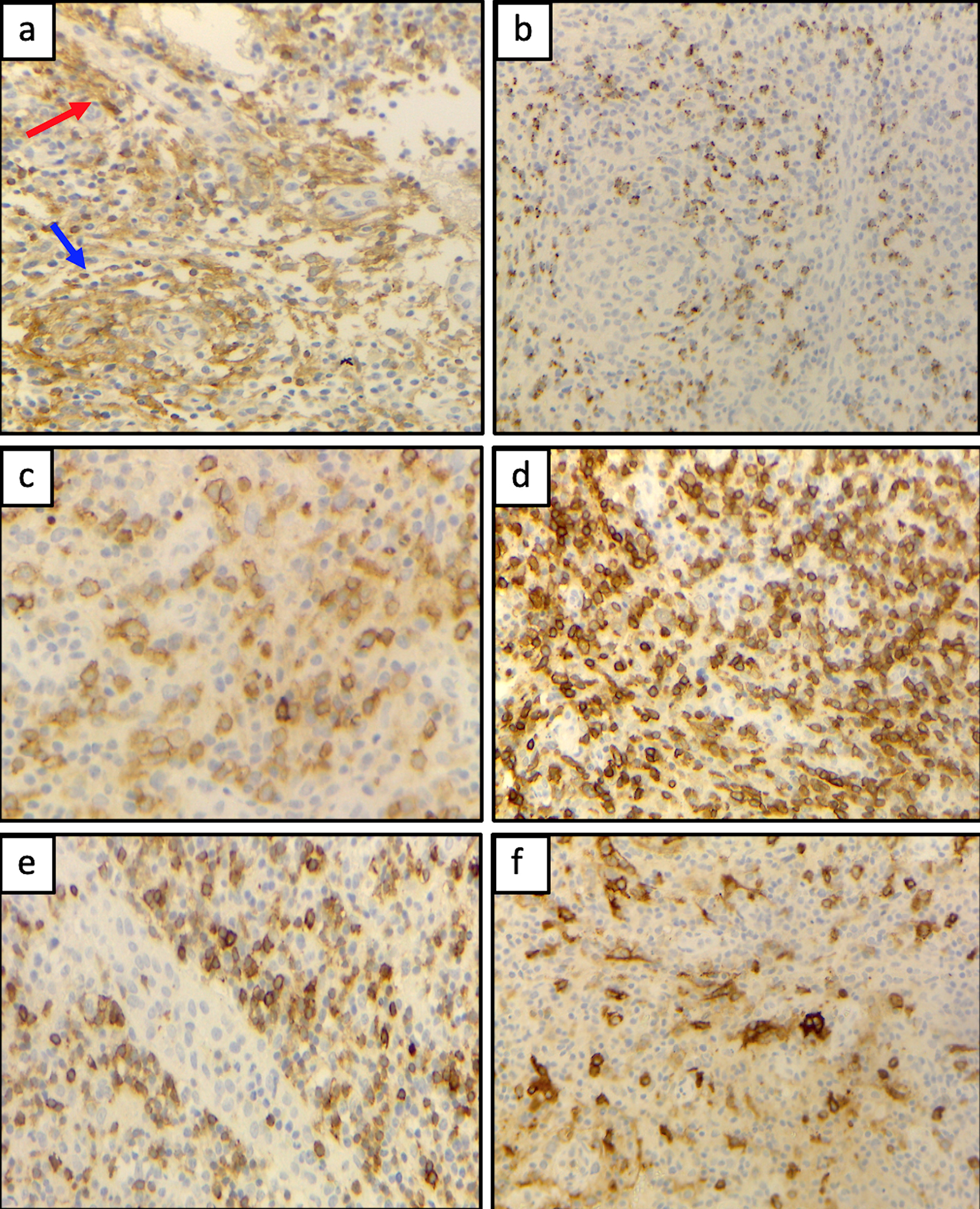 Click for large image | Figure 4. (a) Small to medium-sized T CD4+ cells surrounding endothelial venules (red arrow) and a proliferation of follicular dendritic cells (blue arrow) were variably detected. (b) Accompanying polymorphic inflammatory cells and CD8+ granzyme+ T cells were observed. The atypical T-cell population expressed CD10+ (c), CD5+ (d), with partial expression of CD7 (e), and CD30 (f). |
The T-cell receptor gene rearrangement was detected by a molecular analysis. At presentation Epstein-Barr (EBV) DNA was detected with a viral load of 406 U/mL (cutoff value < 104 U/mL).
The BM involvement was also demonstrated by flow cytometry, with a percentage of nTFHL-AI cells of 0.02%.
Front-line therapy with cyclophosphamide, doxorubicin, vincristine, prednisone and etoposide (CHEOP) for six cycles followed by autologous stem cell transplantation (ASCT) was planned. After the second CHEOP cycle, the patient presented progressive disease, salvage therapy with bendamustine, gemcitabine and vinorelbine (BeGEV) combination was started in order to obtain disease control and autologous stem cell mobilization. Given the clinical evidence of disease progression and stem cell mobilization failure, a third-line therapy with brentuximab vedotin (BV) was started. After the second BV cycle, a computed tomography scan documented recurrent diffuse lymphadenopathy, suggesting again disease progression. The patient was finally treated with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD) and achieved complete remission after the first cycle. Matched unrelated donor (MUD) allogeneic hematopoietic transplantation was performed and managed to maintain disease remission. During chemotherapy, EBV viral load was not detectable anymore.
| Discussion | ▴Top |
The diagnosis of nTFHL-AI can be challenging and requires a specific immunophenotypic. The use of high-sensitivity flow cytometry panel including anti-TRBC1 antibody (JOVI-1) allows to disclose small circulating populations with nTFHL-AI phenotype, as described in this case report.
nTFHL-AI, previously known as angioimmunoblastic T-cell lymphoma, is the second most common peripheral T-cell lymphoma in Western countries. It is an aggressive neoplasm, whose features include skin rash, fever, generalized lymphadenopathy, polyclonal hypergammaglobulinemia, and central or peripheral neurologic manifestations. Some clinical features are more related to the accompanying immune dysfunction than to tumor growth [10]. Polyclonal hypergammaglobulinemia occurs in approximately 50% of nTFHL-AI cases, and autoimmune hemolytic anemia can be present at diagnosis in 19% of patients [11].
In our case, we reported both autoimmune hemolytic anemia and hypergammaglobulinemia with marked polyclonal peripheral plasmacytosis, detected by flow cytometry. B-cell or plasma cell expansion is common in nTFHL-AI and in some cases can lead to misinterpretation as B-cell lymphoma, myeloma, or classic Hodgkin lymphoma [4]. The mechanism of polyclonal proliferation of plasma cells in the peripheral blood of patients with nTFHL-AI is probably multifactorial. EBV can be detected in 41% of nTFHL-AI cases and may contribute to the reactive plasma cell proliferation [5, 12].
nTFHL-AI is a many-faced lymphoma [13], therefore after first excluding a B-cell lymphoma, we have focused on the T population with a high-sensitivity MFC panel including CD3, CD4, CD5, CD7, CD8, and CD10 markers. In most cases, the abnormal nTFHL-AI cells are known to express CD2, CD4, and CD5, with negativity for CD8 [14]. In nTFHL-AI, the most frequent T-cell immunophenotypic aberrancies include the loss or the reduced intensity of surface CD3 and CD7, with aberrant CD10 expression [15]. Singh et al reported that the absence of CD3 on the surface (sCD3) of CD4+ T cells has a high positive predictive value for the diagnosis of nTFHL-AI [8]. However, in this nTFHL-AI case, the T cells were positive for sCD3 with strong intensity of CD5 and a reduced expression of CD4, if compared to normal CD4+ T cells. In addition, aberrant CD10 expression and reduced intensity of CD7 were also present. The strong expression of CD5 on nTFHL-AI cells was reported by Chen et al in a retrospective analysis of immunophenotypic features in 15 cases of nTFHL-AI [16]; however, the concurrent immunophenotypic aberrancies reported in the present case were not previously detected. Immunophenotyping features identified in the peripheral blood were confirmed by IHC on excised lymph node sections. The expression of sCD3 led us to use anti-TRBC1 antibody (JOVI-1), to investigate the T-cell clonal restriction of the abnormal nTFHL-AI cells. The anti-TRBC1 antibody (JOVI-1) has recently been identified as an immunophenotypic marker potentially useful for the assessment of T-cell clonality in samples from patients with suspected T-cell chronic lymphoproliferative disorders [17]. This antibody is specific for human TRBC1. During the αβ-TCR rearrangement, the choice between the two constant regions C1 and C2 becomes mutually exclusive, consequently all cells derived from a T cell undergoing clonal expansion show a positive (unimodal) or negative signal for TRBC1 [17, 18]. Capone et al demonstrated that the use of TRBC1 is useful in identifying small mature T-cell clones of uncertain significance (T-CUS), confirming the high sensitivity of the method [19]. In this case report, the high-sensitivity MFC analysis, including TRBC1, disclosed a small circulating population of atypical T cells (0.07%). According to data reported by Loghavi et al, the aberrant nTFHL-AI cells, detected by MFC represented 0.5-80% of lymphocytes (median 8.5%) and 0.02% to 67.5% (median 2.5%) of all peripheral white cells [20]. These data suggested that using appropriate MFC panels, small aberrant nTFHL-AI cells might be accurately detected, as we have reported.
In this case, off-label therapy with BV was started in third line, according to Horwitz et al’s study that reported objective responses with BV in 41% of patients with relapsed peripheral T-cell lymphomas, including 54% of nTFHL-AI patients [21]. However, after the second BV cycle, diffuse lymphadenopathy developed, suggesting disease progression.
Conclusion
Although nTFHL-AI is a subtype of peripheral T-cell lymphomas, cells expressing the typical immunophenotypic features of nTFHL-AI are seldom identified in peripheral blood samples. In this case, the unexpected detection of a small circulating population of nTFHL-AI cells by high-sensitivity MFC has prompted an extensive diagnostic workup leading rapidly to the correct diagnosis. Therefore, an integrated approach, including clinical data, histologic and molecular findings, and an appropriate high-sensitivity immunophenotypic analysis, is essential to reach an accurate and rapid diagnosis of nTFHL-AI cells, allowing the prompt start of intensive chemotherapy.
Learning points
The diagnosis of nTFHL-AI can be challenging and requires the use of a specific immunophenotypic panel. The peripheral blood involvement in nTFHL-AI seems rare and has not been frequently addressed in the literature. A high-sensitivity flow cytometry panel including anti-TRBC1 antibody JOVI-1 is useful to disclose the small circulating population with nTFHL-AI phenotype.
Acknowledgments
None to declare.
Financial Disclosure
There is no external funding for this article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Informed Consent
Written informed consent was obtained from the patient for publication of this case report.
Author Contributions
A. Gatti and S. Franceschetti contributed to the conception and design of the study and wrote the manuscript. M. Draisci and A. Corso assisted in the diagnosis and therapy of patients. All authors contributed to the manuscript revision, read and approved the submitted version.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Attygalle AD, Chan JKC, Coupland SE, Du MQ, Ferry JA, de Jong D, Gratzinger D, et al. What is new in the 5th edition of the World Health Organization classification of mature B and T/NK cell tumors and stromal neoplasms? J Hematop. 2024;17(2):71-89.
doi pubmed - Iannitto E, Ferreri AJ, Minardi V, Tripodo C, Kreipe HH. Angioimmunoblastic T-cell lymphoma. Crit Rev Oncol Hematol. 2008;68(3):264-271.
doi pubmed - Federico M, Rudiger T, Bellei M, Nathwani BN, Luminari S, Coiffier B, Harris NL, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31(2):240-246.
doi pubmed - Xie Y, Jaffe ES. How I diagnose angioimmunoblastic T-cell lymphoma. Am J Clin Pathol. 2021;156(1):1-14.
doi pubmed - Ahsanuddin AN, Brynes RK, Li S. Peripheral blood polyclonal plasmacytosis mimicking plasma cell leukemia in patients with angioimmunoblastic T-cell lymphoma: report of 3 cases and review of the literature. Int J Clin Exp Pathol. 2011;4(4):416-420.
pubmed - Cho YU, Chi HS, Park CJ, Jang S, Seo EJ, Huh J. Distinct features of angioimmunoblastic T-cell lymphoma with bone marrow involvement. Am J Clin Pathol. 2009;131(5):640-646.
doi pubmed - Jain G, Kumar C, Malhotra A, Mallick SR, Bakhshi S, Chopra A. Peripheral blood involvement in angioimmunoblastic T-cell lymphoma: a case report and review of the literature. Am J Blood Res. 2020;10(5):257-265.
pubmed - Singh A, Schabath R, Ratei R, Stroux A, Klemke CD, Nebe T, Florcken A, et al. Peripheral blood sCD3(-) CD4(+) T cells: a useful diagnostic tool in angioimmunoblastic T cell lymphoma. Hematol Oncol. 2014;32(1):16-21.
doi pubmed - Mosalpuria K, Bociek RG, Vose JM. Angioimmunoblastic T-cell lymphoma management. Semin Hematol. 2014;51(1):52-58.
doi pubmed - Sachsida-Colombo E, Barbosa Mariano LC, Bastos FQ, Rassi AB, Lage LA, Barreto A, Siqueira S, et al. A difficult case of angioimmunoblastic T-cell lymphoma to diagnose. Rev Bras Hematol Hemoter. 2016;38(1):82-85.
doi pubmed - Mukherjee T, Dutta R, Pramanik S. Aggressive angioimmunoblastic T cell lymphomas (AITL) with soft tissue extranodal mass varied histopathological patterns with peripheral blood, bone marrow, and splenic involvement and review of literature. Indian J Surg Oncol. 2018;9(1):11-14.
doi pubmed - Huppmann AR, Roullet MR, Raffeld M, Jaffe ES. Angioimmunoblastic T-cell lymphoma partially obscured by an Epstein-Barr virus-negative clonal plasma cell proliferation. J Clin Oncol. 2013;31(2):e28-30.
doi pubmed - Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood. 2017;129(9):1095-1102.
doi pubmed - Chiba S, Sakata-Yanagimoto M. Advances in understanding of angioimmunoblastic T-cell lymphoma. Leukemia. 2020;34(10):2592-2606.
doi pubmed - Stacchini A, Demurtas A, Aliberti S, Francia di Celle P, Godio L, Palestro G, Novero D. The usefulness of flow cytometric CD10 detection in the differential diagnosis of peripheral T-cell lymphomas. Am J Clin Pathol. 2007;128(5):854-864.
doi pubmed - Chen W, Kesler MV, Karandikar NJ, McKenna RW, Kroft SH. Flow cytometric features of angioimmunoblastic T-cell lymphoma. Cytometry B Clin Cytom. 2006;70(3):142-148.
doi pubmed - Maciocia PM, Wawrzyniecka PA, Philip B, Ricciardelli I, Akarca AU, Onuoha SC, Legut M, et al. Targeting the T cell receptor beta-chain constant region for immunotherapy of T cell malignancies. Nat Med. 2017;23(12):1416-1423.
doi pubmed - Novikov ND, Griffin GK, Dudley G, Drew M, Rojas-Rudilla V, Lindeman NI, Dorfman DM. Utility of a simple and robust flow cytometry assay for rapid clonality testing in mature peripheral T-cell lymphomas. Am J Clin Pathol. 2019;151(5):494-503.
doi pubmed - Capone M, Peruzzi B, Palterer B, Bencini S, Sanna A, Puccini B, Nassi L, et al. Rapid evaluation of T cell clonality in the diagnostic work-up of mature T cell neoplasms: TRBC1-based flow cytometric assay experience. Transl Oncol. 2022;26:101552.
doi pubmed - Loghavi S, Wang SA, Medeiros LJ, Jorgensen JL, Li X, Xu-Monette ZY, Miranda RN, et al. Immunophenotypic and diagnostic characterization of angioimmunoblastic T-cell lymphoma by advanced flow cytometric technology. Leuk Lymphoma. 2016;57(12):2804-2812.
doi pubmed - Horwitz SM, Advani RH, Bartlett NL, Jacobsen ED, Sharman JP, O'Connor OA, Siddiqi T, et al. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood. 2014;123(20):3095-3100.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.