| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com |
Case Report
Volume 16, Number 4, April 2025, pages 135-139
Noncommunicating Hydrocephalus Caused by Aseptic Meningitis Can Be Treated With Endoscopic Third Ventriculostomy
Ayumu Nittaa, Yasuo Sasagawab, c, Mitsutoshi Nakadab
aDepartment of Neurosurgery, Toyama City Hospital, Toyama-shi, Toyama, 939-8511, Japan
bDepartment of Neurosurgery, Kanazawa University, Kanazawa, Ishikawa, 920-8641, Japan
cCorresponding Author: Yasuo Sasagawa, Department of Neurosurgery, Kanazawa University, Ishikawa, 920-8641, Japan
Manuscript submitted January 16, 2025, accepted April 8, 2025, published online April 22, 2025
Short title: ETV for Noncommunicating Hydrocephalus
doi: https://doi.org/10.14740/jmc5109
| Abstract | ▴Top |
Noncommunicating hydrocephalus is a rare complication of aseptic meningitis and is predominantly characterized by the obstruction of cerebrospinal fluid (CSF) absorption. Traditional treatment methods include external ventricular drainage (EVD) and ventriculoperitoneal (VP) shunt. However, endoscopic third ventriculostomy (ETV) might also be effective in the case of aseptic meningitis. A 19-year-old woman presented with fever, lymphadenopathy, and arthritis, followed by headache and vomiting. Magnetic resonance imaging (MRI) showed no hydrocephalus. CSF analysis revealed mild pleocytosis and elevated protein levels, with negative cultures, leading to the diagnosis of aseptic meningitis. After an initial recovery with supportive care, the patient returned a month later with an acute progressive headache and altered consciousness. Computed tomography (CT) revealed lateral and third ventricular enlargement, while MRI showed cerebellar swelling and foraminal adhesions, indicative of obstructive hydrocephalus. ETV was performed through the right anterior horn of the lateral ventricle. Postoperative recovery was uneventful, and the patient remained asymptomatic after steroid treatment. ETV is a viable option for treating noncommunicating hydrocephalus associated with aseptic meningitis, even when CSF malabsorption is present.
Keywords: Aseptic meningitis; Noncommunicating hydrocephalus; Endoscopic third ventriculostomy
| Introduction | ▴Top |
Noncommunicating hydrocephalus is a rare complication of aseptic meningitis. Most cases of hydrocephalus caused by aseptic meningitis are of the communicating type, especially with obstruction of cerebrospinal fluid (CSF) absorption [1]. The precise annual incidence of aseptic meningitis is uncertain, but it is known this condition occurs in all races and ages and tends to be slightly more common in males and children under 1 year old [2]. Meningitis complicates brain herniation in about 6% of cases, and hydrocephalus is one of the possible causes. Brain herniation due to acute obstructive hydrocephalus is life-threatening and requires immediate and appropriate treatment.
The main CSF diversion method for hydrocephalus in aseptic meningitis is external ventricular drainage (EVD) or ventriculoperitoneal (VP) shunt placement. However, these treatments have been associated with complications such as bacterial infections and over drainage, as well as long-term complications such as slit-like ventricle syndrome in VP shunt. Recent studies have suggested that ETV might be an option for hydrocephalus due to meningitis, either noncommunicating or communicating [3, 4]. ETV has the potential to solve the problems of VP shunts and ETV presented above.
In the present report, we describe a case of noncommunicating hydrocephalus caused by aseptic meningitis that was successfully treated with ETV, along with a literature review.
| Case Report | ▴Top |
A 19-year-old woman presented with fever, lymphadenopathy, and arthritis, followed by a headache and vomiting. She had no specific previous medical history. Magnetic resonance imaging (MRI) did not reveal hydrocephalus. CSF analysis revealed leukocytes of 77 cells/µL (normal range (NR): 0 - 5 cells/µL) with 97% mononuclear cells, protein of 178 mg/dL (NR: 10 - 45 mg/dL), and glucose of 49 mg/dL (NR: 50 - 75 mg/dL). CSF culture was negative and aseptic meningitis was diagnosed. The patient was admitted to the hospital, provided supportive care, and discharged once the headache resolved.
One month later, she presented to the emergency department with a headache and an acute progressive alert level of consciousness. CSF analysis revealed leukocytes of 78 cells/µL with 98% mononuclear cells, protein of 770 mg/dL, and glucose of 31 mg/dL. CSF cultures for bacteria, Mycobacterium tuberculosis, and fungi were negative. CSF virus antigen determinations for influenza virus, herpes simplex virus, varicella-zoster virus, cytomegalovirus, Coxsackie virus, and Epstein-Barr virus were negative. Laboratory findings showed soluble interleukin 2 (sIL-2) receptor of 449 U/mL (NR: 122 - 496 U/mL) and angiotensin-converting enzyme (ACE) of 10.5 U/L (NR: 7 - 25 U/L). Antinuclear antibody (ANA) and T-spot were all negative. Computed tomography (CT) revealed enlargement of the lateral and third ventricles and narrowing of the fourth ventricle due to cerebellar swelling (Fig. 1). MRI with gadolinium-enhanced T1 demonstrated enhancement of the occipital lobe surface, cerebellar surface, foramina of Magendie, and Luschka (Fig. 2). We diagnosed the patient with obstructive hydrocephalus caused by cerebellar swelling and adhesions of foramina of Magendie and Luschka. The patient underwent elective ETV via the right anterior horn of the lateral ventricle. Cerebrospinal pressure was 40 cm H2O. The aqueduct was slightly dilated without thinning the tuber cinereum. Because the tuber cinereum was thick and rigid, several punctures were required to create a stoma. An expanding balloon was used for dilation, and CSF was allowed to flow into the prepontine cistern. Figure 3 shows the operative view of this ETV. The patient had an uneventful postoperative course and regained consciousness the day after surgery. The patient was treated for meningitis with antibacterial, antitubercular, and antiviral agents. Based on the results of the CSF culture and virus antigen examination, aseptic meningitis of unknown cause was diagnosed, and methylprednisolone pulse therapy was performed. Figure 4 shows the postoperative MRI 1 month after surgery. No meningitis or hydrocephalus recurrence was observed for 6 months after steroid tapering.
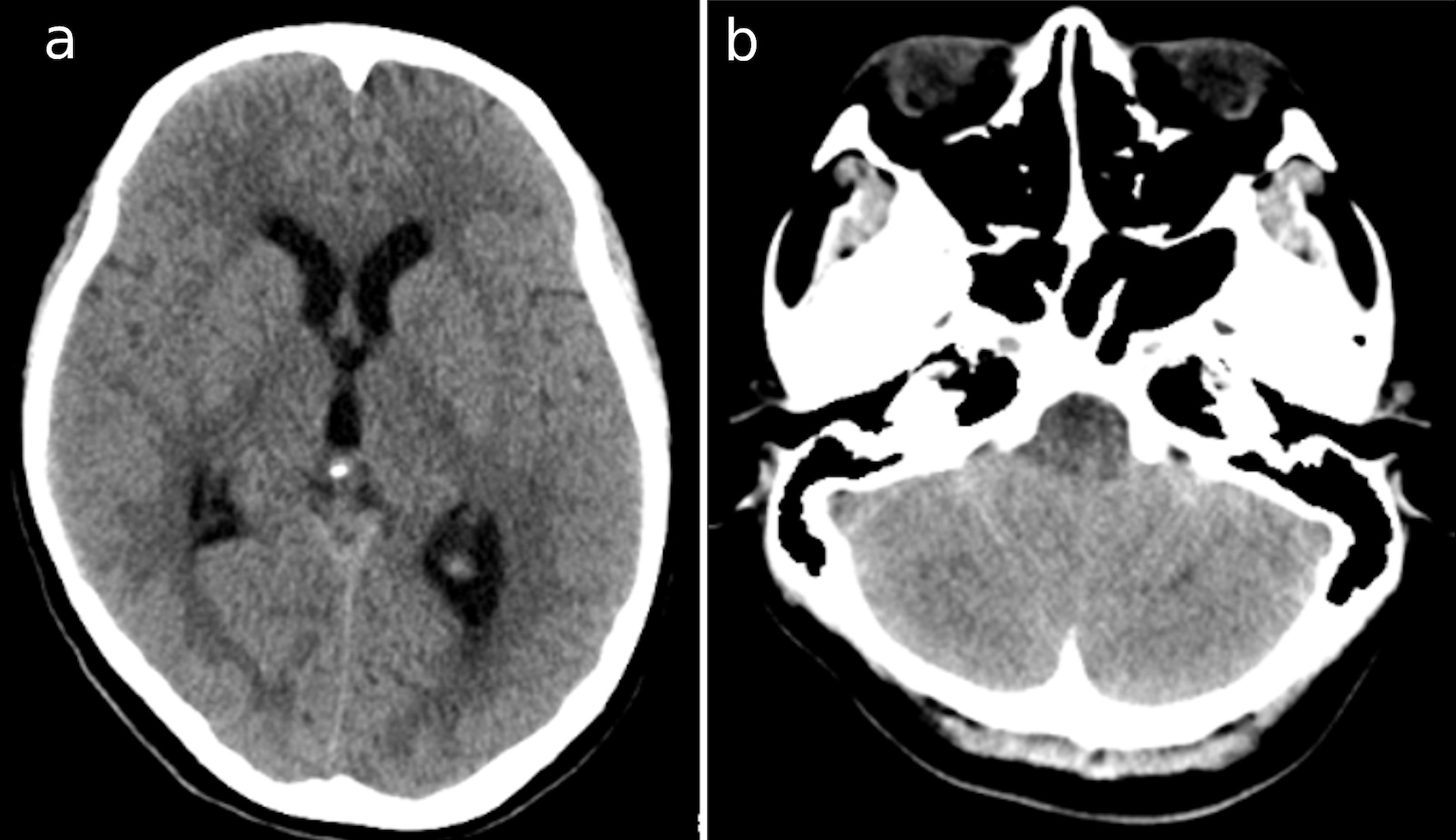 Click for large image | Figure 1. CT images at the second admission show (a) the dilatation of bilateral and third ventricles; and (b) cerebellar swelling. CT: computed tomography. |
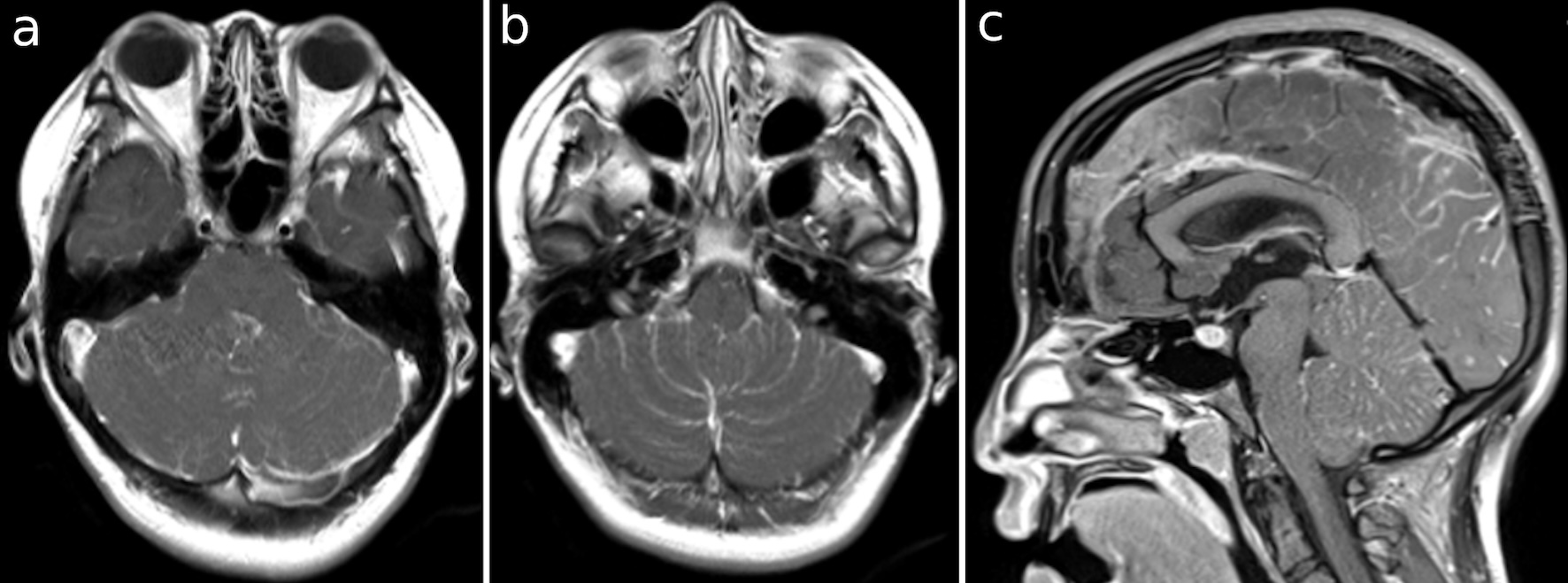 Click for large image | Figure 2. (a-c) T1 images with gadolinium-enhancing occipital lobe and cerebellar surface, foramina of Magendie and Luschka. |
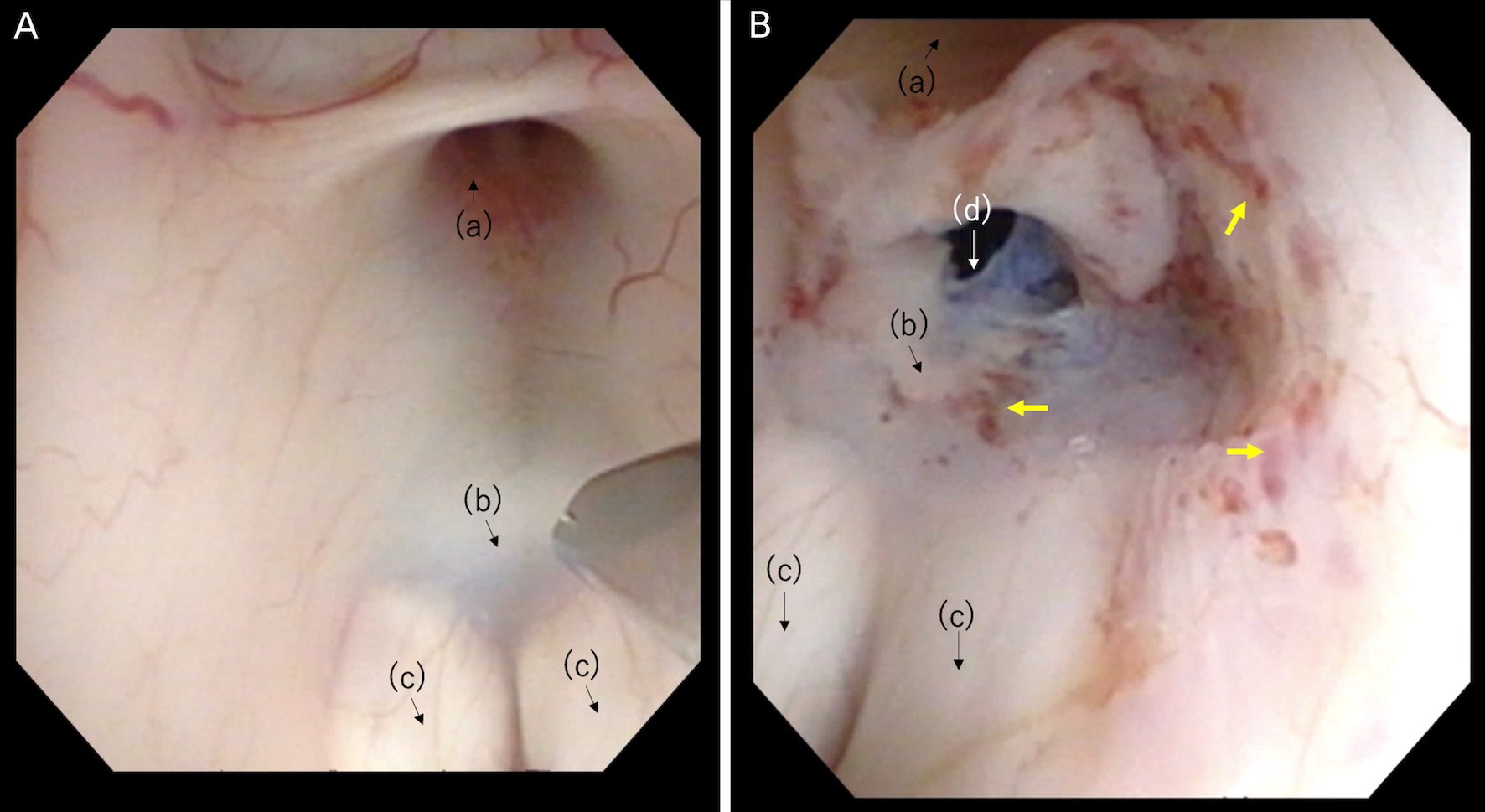 Click for large image | Figure 3. (A) Thick tuber cinereum before puncture. (B) After the expansion of the stoma on the floor of the third ventricle. Anatomical structures: (a) infundibular recess; (b) tuber cinereum; (c) mamillary bodies; (d) stoma. The yellow arrows show dot bleeding by mechanical injury. |
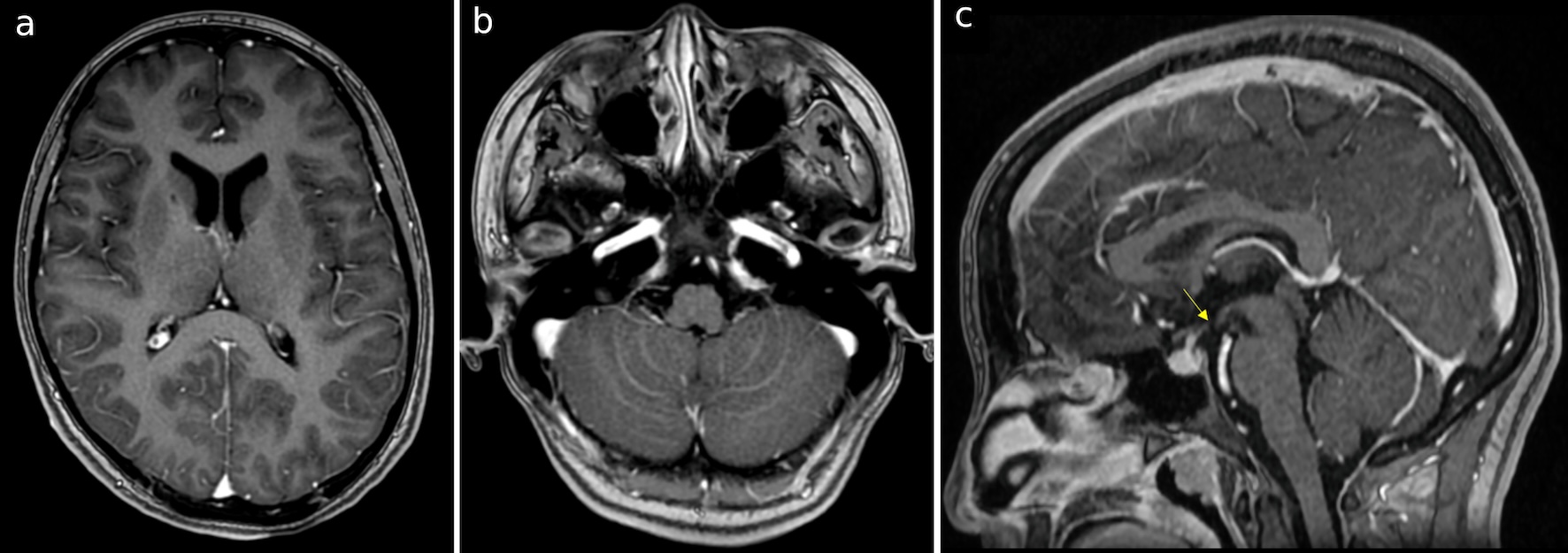 Click for large image | Figure 4. (a-c) T1 images with gadolinium 1 month after the surgery, demonstrating the improvement of cerebellar edema and shrinkage of ventricles. There was no enhancement of the foramina of Magendie and Luschka. The yellow arrow shows the stoma perforated in ETV. |
| Discussion | ▴Top |
Noncommunicating hydrocephalus is rare in aseptic etiology, which is associated with bacterial or tuberculous meningitis, and can result in the obstruction of the outflow tract at the foramen of Monro, third ventricle, foramina of Luschka, or Magendie [5]. Ma et al reported that among 19,643 autoimmune disease cases with concomitant hydrocephalus, 19 were identified, and two of these were diagnosed as obstructive hydrocephalus [1]. The mechanism of noncommunicating hydrocephalus with meningitis includes stenosis of the midbrain aqueduct, adhesion, membrane formation, and obstruction by granulation. In the present case, the cause of hydrocephalus might have been attributed to the narrowing of the outflow tract due to cerebellar edema and obstruction of the foramen of Luschka and Magendie. Acute noncommunicating hydrocephalus leads the patient to life-threatening status unless a quick diagnosis and the right treatments including CSF-diverting operation and medication for meningitis are achieved.
The causes of aseptic meningitis can be classified as infectious or non-infectious [6]. Infectious causes are predominantly viral but can also include bacterial, fungal, and parasitic. Non-infectious causes were classified into three categories: systemic diseases (e.g., sarcoidosis, systemic lupus erythematosus, and vasculitis), drug-induced diseases (e.g., non-steroidal anti-inflammatory drugs (NSAIDs) and antibiotics), and tumor meningitis. About 35% to 70% of aseptic meningitis remains of unknown etiology despite diagnostic examinations, sometimes termed “idiopathic” [2]. Drug treatment should be cause-specific; however, if the etiology is unknown, the initial treatment may include broad-spectrum antibacterial agents, antituberculosis drugs, and steroids until various examination results are available [7]. In the present case, the etiology was unknown despite bacterial and viral polymerase chain reaction and collagen disease tests.
CSF diversion to meningitis hydrocephalus may be a temporary EVD and VP shunt. Classically, ETV is indicated for obstructive hydrocephalus in the absence of absorption disorders or cerebrospinal fluid overproduction. However, ETV has been shown to be effective even in conditions complicated by absorption disorders, such as tuberculous meningitis. Some studies have reported that ETV has lower failure rates than VP shunts [3].
Compared to EVD, ETV reduces the risk of infection if prolonged drainage and multiple surgical procedures due to drain replacement are necessary. Patients with EVD may experience prolonged immobilization, and ETV allows them to avoid this. If the level of consciousness is severely depressed, EVD is rather necessary to reduce intracranial pressure [4]. Compared with VP shunts, it can prevent a shunt-dependent state even after the obstruction has been resolved, and diversion is no longer required. Even if a shunt is needed, ETV can prevent long-term complications, such as over-drainage, retrograde bacterial infections from the abdomen, and slit-like ventricle syndrome [8]. Elevation of the cellular protein count in the CSF, which is often exhibited in acute meningitis, may lead to reoperation due to obstruction of the VP shunt [9]. We chose ETV in the present case to avoid the failure risk if a VP shunt was required.
In cases of ETV failure, other CSF diversions, such as EVD or shunts, are needed. Careful neurological examinations and repeated CT follow-ups are required. Malabsorption does not preclude the choice of ETV; however, the risk of ETV failure in aseptic meningitis remains uncertain. Infants and pediatrics are risk factors for failure according to the ETV success score [8].
It should be highlighted that ETV in the acute phase of meningitis has specific risks different from those in the chronic phase. Thickening of the floor of the third ventricle, caused by inflammation, makes it difficult to identify the anatomical structures (e.g., mammillary bodies) [4]. The rigidity of the floor also increases the difficulty of perforating the ventricle, resulting in vascular injury and incomplete communication [10]. ETV in the inflammatory state should be performed by a well-experienced operator to prevent operative complications.
Conclusion
We described a rare case of noncommunicating hydrocephalus caused by aseptic meningitis. ETV should be considered as a treatment option for noncommunicating hydrocephalus with CSF malabsorption like aseptic meningitis in the acute phase.
Learning points
Aseptic meningitis can cause noncommunicating hydrocephalus with absorption disorder in the acute phase. ETV and appropriate medical treatment is one of the solutions for hydrocephalus without complications associated with other CFS diversions.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent for publication of this case report was obtained from the participant.
Author Contributions
Ayumu Nitta contributed to writing the manuscript. Yasuo Sasagawa and Mitsutoshi Nakada reviewed and edited the manuscript before submission.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ACE: angiotensin-converting enzyme; ANA: antinuclear antibody; CSF: cerebrospinal fluid; CT: computed tomography; ETV: endoscopic third ventriculostomy; EVD: external ventricular drainage; MRI: magnetic resonance imaging; NR: normal range; sIL-2: soluble interleukin 2; VP: ventriculoperitoneal
| References | ▴Top |
- Ma B, Wu H, Yin H, Chang J, Wang L, Wang R, Ma W, et al. Management of hydrocephalus associated with autoimmune diseases: a series of 19 cases. Autoimmunity. 2017;50(7):422-427.
doi pubmed - Kaur H, Betances EM, Perera TB. Aseptic meningitis. In: StatPearls. Treasure Island (FL) ineligible companies. 2025.
pubmed - Aranha A, Choudhary A, Bhaskar S, Gupta LN. A randomized study comparing endoscopic third ventriculostomy versus ventriculoperitoneal shunt in the management of hydrocephalus due to tuberculous meningitis. Asian J Neurosurg. 2018;13(4):1140-1147.
doi pubmed - Figaji AA, Fieggen AG. Endoscopic challenges and applications in tuberculous meningitis. World Neurosurg. 2013;79(2 Suppl):S24.e29-14.
doi pubmed - Maller VV, Gray RI. Noncommunicating hydrocephalus. Semin Ultrasound CT MR. 2016;37(2):109-119.
doi pubmed - Tattevin P, Tchamgoue S, Belem A, Benezit F, Pronier C, Revest M. Aseptic meningitis. Rev Neurol (Paris). 2019;175(7-8):475-480.
doi pubmed - Putz K, Hayani K, Zar FA. Meningitis. Prim Care. 2013;40(3):707-726.
doi pubmed - Kulkarni AV, Drake JM, Mallucci CL, Sgouros S, Roth J, Constantini S, Canadian Pediatric Neurosurgery Study G. Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. J Pediatr. 2009;155(2):254-259.e251.
doi pubmed - Paliwal VK, Garg RK. Hydrocephalus in tuberculous meningitis - pearls and nuances. Neurol India. 2021;69(Supplement):S330-S335.
doi pubmed - Abdala-Vargas NJ, Cifuentes-Lobelo HA, Ordonez-Rubiano E, Patino-Gomez JG, Villalonga JF, Lucifero AG, Campero A, et al. Anatomic variations of the floor of the third ventricle: Surgical implications for endoscopic third ventriculostomy. Surg Neurol Int. 2022;13:218.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.