| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com |
Case Report
Volume 16, Number 1, January 2025, pages 37-42
Peritonitis After Endometrial Cytology in a Woman With Hydrosalpinx Caused by Chronic Chlamydia trachomatis Infection
Haruka Minoyamaa, Kazuhide Hidab, Erisa Fujiia, Shun-ichi Ikedaa, c
aDepartment of Gynecology, Tokyo Takanawa Hospital, Tokyo, Japan
bDepartment of Obstetrics and Gynecology, Kohseichuo General Hospital, Tokyo, Japan
cCorresponding Author: Shun-ichi Ikeda, Department of Gynecology, Tokyo Takanawa Hospital, Tokyo 108-8606, Japan
Manuscript submitted October 4, 2024, accepted November 29, 2024, published online December 21, 2024
Short title: Chlamydial Peritonitis Post-Endometrial Test
doi: https://doi.org/10.14740/jmc4344
| Abstract | ▴Top |
Some women infected with Chlamydia trachomatis (CT) are asymptomatic, while others experience lower abdominal discomfort when the inflammatory process extends to the fallopian tubes. Without treatment, salpingitis can progress to pelvic peritonitis and subsequently, peritonitis in the upper abdomen, a condition known as Fitz-Hugh-Curtis syndrome, in some cases. A nucleic acid amplification assay is required for diagnosing CT infection. However, this assay may yield a negative result even in the presence of CT infection. This report presents a case of a 45-year-old woman with a history of hydrosalpinx and no history of lower abdominal pain who underwent endometrial cytology at a local gynecology clinic because of irregular bleeding. The following day, she developed peritonitis. A nucleic acid amplification assay for CT yielded a negative result at the onset of peritonitis. Hence, the patient received tazobactam/piperacillin as a treatment option. However, this drug was ineffective. Subsequently, the patient was found to be positive for serum CT IgG and IgA antibodies. Her peritonitis could have developed as a result of endometrial cytology performed in the presence of a chronic CT infection in the uterus; through this procedure, CT-infected endometrial cells may have spread into the abdominal cavity via the fallopian tubes. Nevertheless, the addition of minocycline hydrochloride markedly improved the symptoms of peritonitis. This case shows that when dealing with chronic CT infections in the female internal genitalia, the standard nucleic acid amplification testing screening test for CT might not be entirely effective in detecting the infection. Additionally, it is important to recognize that in cases of chronic CT infection of the uterus that involved genital bleeding, examining the uterine cavity could lead to peritonitis in a short timeframe.
Keywords: Chlamydia trachomatis; Peritonitis; Nucleic acid; Amplification; Serum chlamydia antibody; Hydrosalpinx
| Introduction | ▴Top |
Chlamydia trachomatis (CT) was previously associated with the eye infection known as trachoma, but it is now recognized as a sexually transmitted infection. When transmitted through sexual intercourse, men may experience urethritis, whereas women may develop cervicitis. Often, symptoms go unnoticed, and untreated cases can lead to complications. Additionally, men might suffer from epididymitis and women from salpingitis. For women, this can result in serious conditions like tubal epithelial damage, pelvic adhesions, tubal pregnancy, and infertility. If a woman is pregnant and still infected with chlamydia, it can lead to chorioamnionitis, potentially causing miscarriage or premature delivery.
Additionally, during childbirth, the infection can result in neonatal conjunctivitis and pneumonia due to birth canal infection. Another consequence is pelvic inflammatory disease (PID), where inflammation spreads from the vagina to the uterus, fallopian tubes, and pelvis. PID can cause widespread abdominal peritonitis, including perihepatitis, a condition referred to as Fitz-Hugh-Curtis syndrome [1, 2].
Nucleic acid amplification testing (NAAT), which directly detects the pathogen’s nucleic acid, is the preferred method for detecting CT infection. The United States Preventive Services Task Force and the Centers for Disease Control and Prevention even recommend NAAT for CT screening because of its high sensitivity and specificity [3, 4]. Furthermore, serological tests reportedly cannot replace NAAT in directly detecting CT.
However, in the present case, we encountered a clinical situation wherein a patient suddenly developed peritonitis of the entire abdomen, and serological testing was more useful than NAAT in diagnosing CT.
This patient was started on tazobactam/piperacillin because DNA-based CT was negative in NAAT. However, her clinical symptoms did not improve. Later, she was found to be positive for anti-CT IgA antibodies, and after treatment with minocycline hydrochloride, her clinical symptoms markedly improved.
| Case Report | ▴Top |
Investigations
A 45-year-old woman presented to a local gynecology clinic with a complaint of heavy menstrual bleeding that persisted for more than 10 days. She had no previous findings of lower abdominal pain suspicious of PID. Transvaginal ultrasound revealed a cyst measuring 60 mm in diameter located in the right uterine adnexa. The endometrium was 5.0 mm thick. The size of the left adnexa was normal, and no tenderness was observed while applying the transvaginal ultrasound probe to the bilateral uterine adnexa.
The physician proceeded to perform a Pap smear and endometrial cytology. When attempting to perform endometrial cytology, the physician encountered difficulty in inserting the endometrial cytology instrument. Consequently, a stylet was inserted into the endometrial cytology instrument for the procedure.
On the following day, the patient began to experience abdominal discomfort, which intensified over time. She then consulted the same gynecologist. At that time, she presented with abdominal pain and a fever of 38 °C. Transvaginal ultrasound detected a moderate amount of fluid in the Douglas fossa, but the cyst in the right uterine adnexa remained at 60 mm in diameter. The clinic physician postulated that perforation of the uterus during endometrial cytology caused the damage to surrounding organs. The patient was then transported by ambulance to the emergency room of a local hospital.
Diagnosis
Upon arrival at the emergency department, the patient exhibited a fever of 38.5 °C. Her blood pressure was 110/70 mm Hg, with persisting genital bleeding. The entire abdomen exhibited marked tension, and rebound tenderness was observed. Venous blood analysis, bacterial cultures of venous blood, urine, and vaginal secretions, and DNA-based NAAT on CT (the Roche cobas 6800/8800 CT/NG Assay) were performed.
Blood analysis results were as follows: white blood cell (WBC) count, 7,240/µL; C-reactive protein (CRP) level, 23.94 mg/dL; hemoglobin (Hb) concentration, 9.9 g/dL; and platelet count, 18.9 × 104/µL. Subsequently, the bacterial culture and CT NAAT results for the aforementioned specimens were negative. A contrast-enhanced computed tomography scan revealed the presence of thickening of the small intestinal wall, Kerckring’s folds, and small intestinal gas (Fig. 1). Additionally, the right adnexa had a cystic lesion (Fig. 2). The diagnosis on admission was peritonitis and a right adnexal cystic mass.
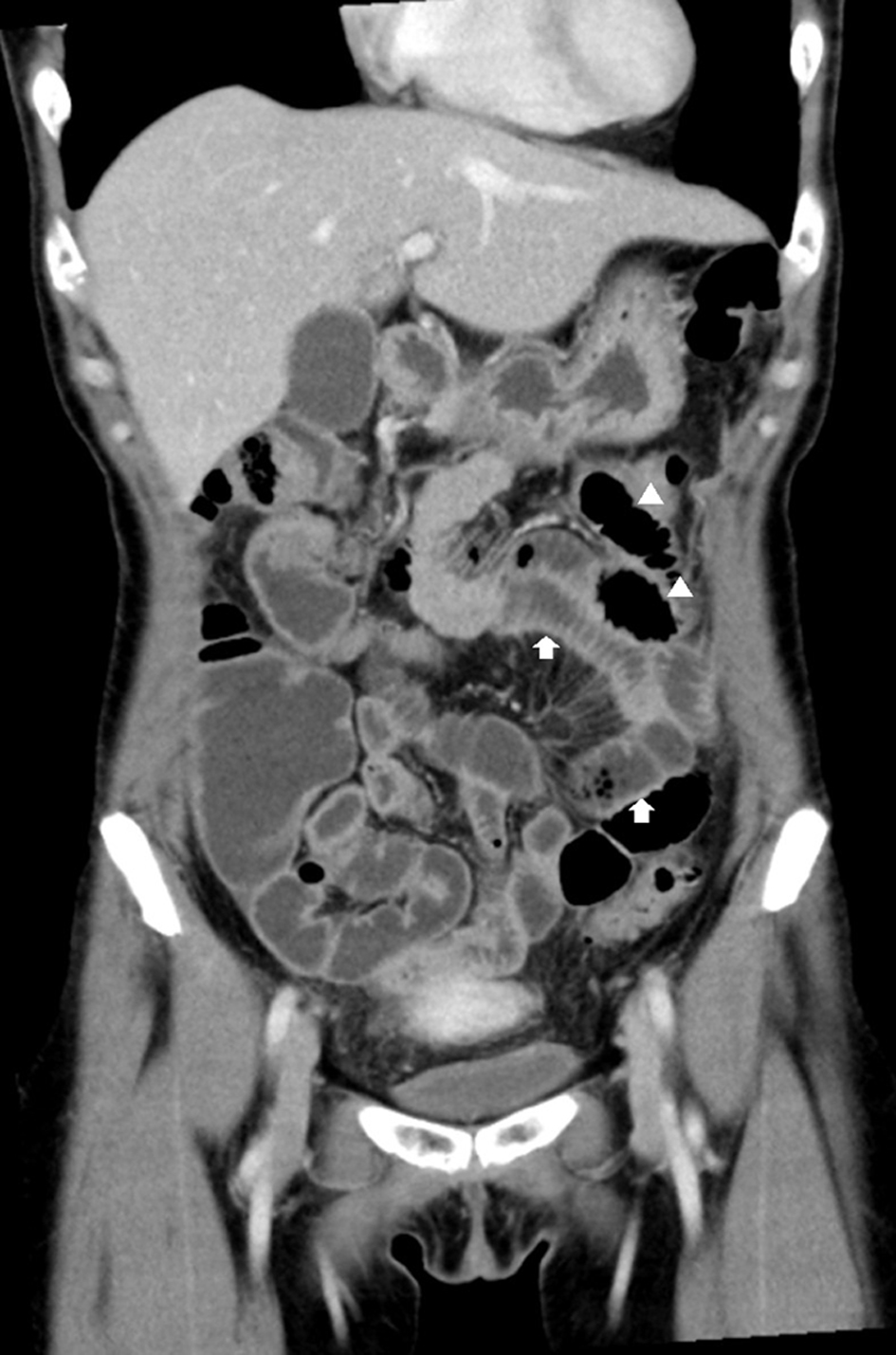 Click for large image | Figure 1. Contrast-enhanced computed tomography of the upper abdomen shows circular folds of Kerckring, thickening of the bowel wall (arrows), and small intestinal loops containing gas (arrowheads). |
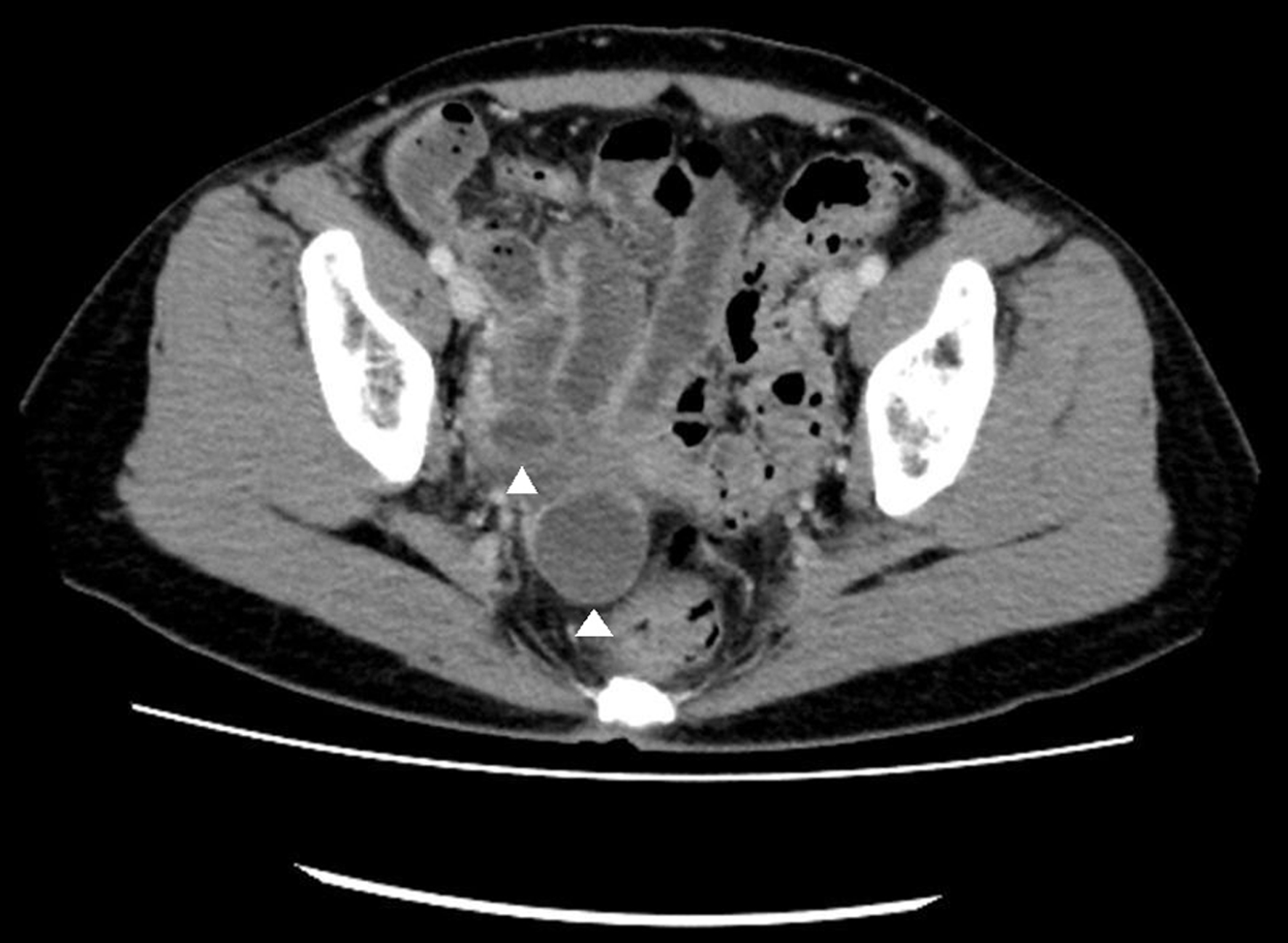 Click for large image | Figure 2. Pelvic contrast-enhanced computed tomography shows a cystic lesion of the right adnexa (arrowheads). |
The contrast-enhanced computed tomography revealed no free air in the abdominal cavity and no visible signs of inflammation in the intrahepatic bile ducts and gallbladder. Transvaginal ultrasonography showed a cyst that was confirmed to not be an abscess and indicated no obvious signs of an adnexal tumor rupture. Consequently, treatment was started for idiopathic peritonitis.
Treatment
On admission day, tazobactam/piperacillin therapy (4.5 g, every 8 h) was initiated on the basis of the diagnosis of peritonitis. On the second day, her fever of over 38 °C persisted.
Two days after antimicrobial therapy initiation, the abovementioned blood test results increased (WBC, 9,550/µL; CRP, 30.62 mg/dL; hemoglobin, 10.3 g/dL; platelets, 190,000/µL), suggesting an augmented inflammatory response since admission.
A contrast-enhanced computed tomography scan of the right adnexa revealed a cystic lesion later confirmed as right tubal hydrops through pelvic magnetic resonance imaging (MRI) with contrast medium on day 3. Hydrosalpinx measured 6.0 × 3.0 cm in diameter and exhibited notable thickening of the fallopian tube wall (Fig. 3).
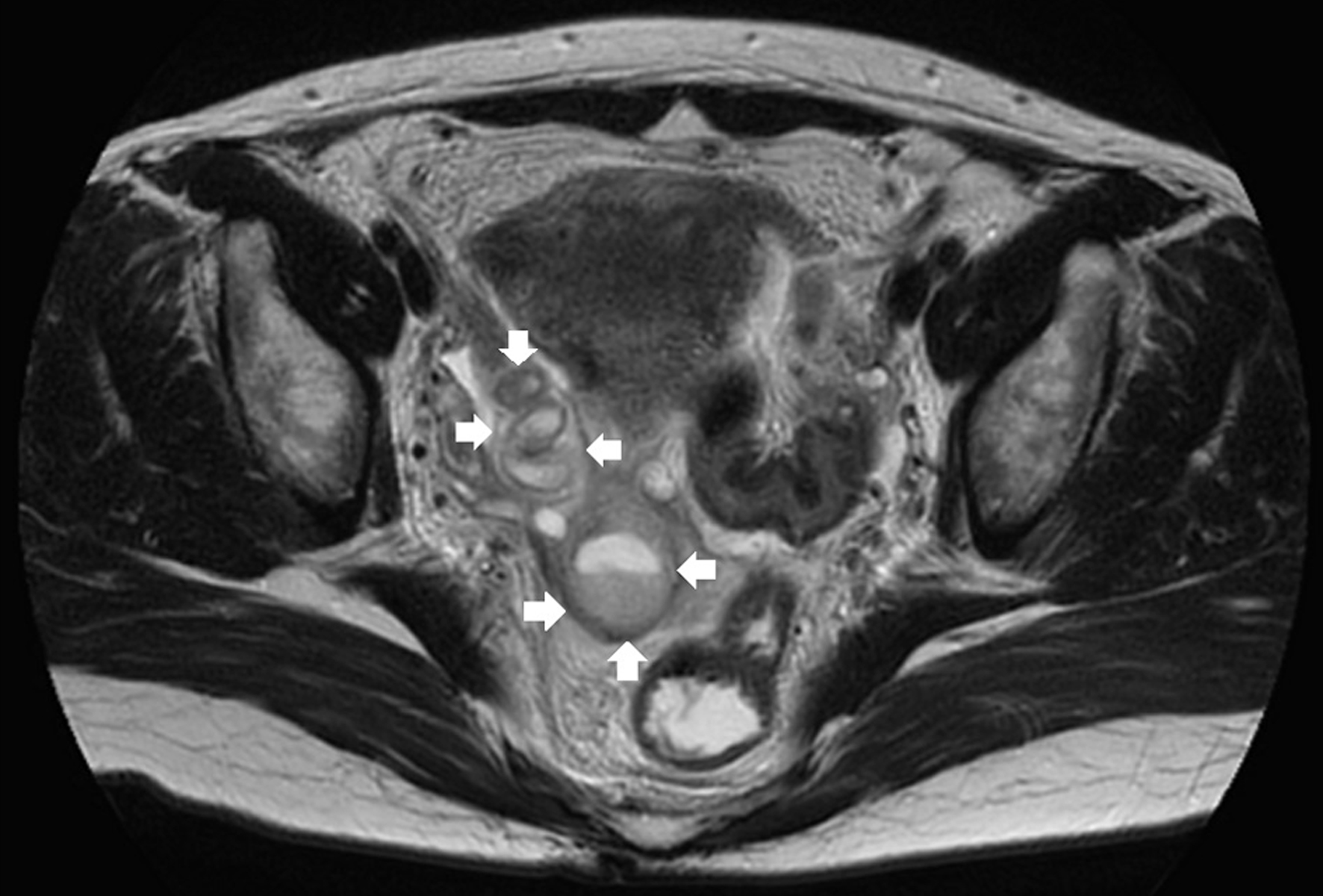 Click for large image | Figure 3. T2-weighted imaging MRI shows a thick-walled tubular lesion in the right adnexa (arrow). MRI: magnetic resonance imaging. |
Given the negative result of the DNA-based NAAT for CT, CT-induced pelvic peritonitis was not initially considered as a potential diagnosis. However, the contrast-enhanced MRI indicated that the cystic lesion in the right adnexa originated from the fallopian tube. Consequently, despite the DNA-based NAAT for CT being negative, CT antibodies were tested to exclude CT infection. On day 3, the cut-off index (COI) of CT antibodies IgG and IgA were found to be 4.89 (positive) and 1.13 (positive), respectively. The positive CT IgA result raised suspicion for a CT infection. On day 4, the patient began receiving intravenous minocycline hydrochloride (100 mg) every 12 h.
If minocycline hydrochloride proved ineffective, we would consider an exploratory laparotomy to exclude the possibility of a digestive tract perforation without free gas [5, 6].
Follow-up and outcomes
On day 6, the patient’s temperature had decreased to 36.5 °C, and his blood analysis results had improved to 6,420/µL for WBC count, 9.8 g/dL for hemoglobin, 189,000/µL for platelet count, and 3.36 mg/dL for CRP.
Minocycline hydrochloride administration was continued until day 10. Four months later, the COI of CT IgG and IgA decreased to 4.80 (positive) and IgA 0.70 (negative). A year after the treatment, the COI levels for CT IgG and IgA were 6.46 (positive) and 0.80 (negative), respectively, and the patient shows no clinical symptoms like abdominal pain.
| Discussion | ▴Top |
CT is a gram-negative bacterium and the most frequently reported sexually transmitted disease in Japan [7]. In women, this infection is often asymptomatic, but if left untreated, it can progress from cervicitis to salpingitis, which can result in infertility, ectopic pregnancy, and PID. Thus, early detection is crucial to prevent complications.
Initially, the patient’s main complaint was genital bleeding, but no symptoms of suspected salpingitis were noted before peritonitis symptoms occurred. This case differs from the typical presentation of CT peritonitis in the following ways. First, a DNA-based NAAT for CT yielded a negative result. Second, after undergoing an endometrial examination (endometrial cytology in this case) to evaluate the cause of the genital bleeding, the patient exhibited peritonitis symptoms within 24 h after the examination. These two characteristics are explained subsequently.
Positive for CT IgA antibodies but negative for DNA-based NAAT on CT
CT NAAT is considered the gold standard for diagnosing CT infection. NAAT can be amplified with a few copies of the target sequence [8]. Nevertheless, DNA-based NAAT must be discernible in certain instances, including the present case. In such instances, the etiology of intrauterine CT infection can be attributed to three factors.
First, chronic persistent infection in the uterus can spread the bacteria into the abdominal cavity, as in this case. Generally, in the acute phase, the number of bacteria increases, resulting in tissue destruction, elevated levels of WBC and CRP (indicators of active inflammation), and severe symptoms such as high fever. During the chronic phase, the immune system partially controls the infection, the number of bacteria decreases, and the symptoms often become milder. Liang et al collected 123 urogenital swabs from outpatients. They tested them for CT, Neisseria gonorrhoeae, and Ureaplasma urealyticum presence using an RNA-based simultaneous amplification test assay and a DNA-based quantitative real-time polymerase chain reaction (qPCR) assay. They found that low pathogen levels can yield false-negative results in PCR testing, in addition to comparing the performance of DNA-based qPCR assay [9]. In other words, if the chronic phase of infection had fewer bacteria than the acute phase, as in the present case, NAAT may not be able to detect CT infection.
Second, the sites for performing NAAT could be more challenging to sample. NAAT can be performed on specimens from various sites, such as urine and vaginal secretions. However, false-negative results may occur when persistent infection is confined to sites that are difficult to access with instruments, such as the uterine fundus or fallopian tubes. In the current case, cells for NAAT were collected from the cervix, but this test may not detect the pathogen because the CT was localized at the base of the uterine cavity.
Third is the timing of specimen collection by NAAT. A high concentration of blood may result in false-negative outcomes. A study of female sex workers in Kenya compared the diagnostic efficacy of three types of NAAT (transcription-mediated amplification (TMA), ligase chain reaction (LCR), and polymerase chain reaction enzyme immunoassay (PCR-EIA)) by using cervical, initial, and intermediate urine specimens collected at varying stages of the menstrual cycle; results revealed that NAAT sensitivity varied by specimen type and menstrual cycle stage [10]. Sensitivity was greater in cervical specimens than in urine specimens and was lower in specimens collected during menstruation than in those collected at other times.
In other words, CT NAAT on specimens with blood contamination can cause false-negative results, even in the presence of CT in the genitalia. If chronic infection is suspected, especially if genital bleeding is frequent, CT infection should not be excluded entirely because of a negative NAAT result, and treatment should be considered according to serum antibody titers.
CT infection stimulates the production of IgG, IgA, and IgM antibodies by plasma cells. Usually, the serum concentrations of specific IgM, IgA, and IgG increase progressively between 5 and 20 days after infection. IgM, a marker of acute infection, is typically detected on approximately day 5, and IgA is increased after approximately 10 days, with a further increase after day 10. The presence of IgM antibodies indicates an early stage of viral infection. IgG antibodies to CT rise in the first month after infection and persist for several years, whereas IgA antibodies to CT rise in the second week after infection and disappear within 6 months after healing [11].
According to Muntaha et al, seropositivity for IgA is high in CT-infected patients, indicating a state of chronic infection [12]. Den Hartog et al reported similar results in CT infection, with significantly higher IgA levels in women with lesions in the fallopian tubes than in those without such lesions. Therefore, a chronic state during CT infection may be associated with increased IgA [13].
Peritonitis symptoms developing within 24 h after endometrial cytology despite no lower abdominal pain or other findings of PID initially
The patient developed peritonitis within 24 h after endometrial cytology examination. The rapid onset may have resulted from three conditions: 1) the original uterine bleeding caused blood to flow back into the pelvic cavity through the fallopian tubes; 2) the endometrial cytology artificially stripped the endometrial cells and released multiple CT-infected endometrial cells; and 3) CT infected the peritoneum and intestinal tract of the abdominal cavity because of the physiological intraperitoneal inflow of ascites. The combination of these three conditions may have caused peritonitis in a short period.
In this case, we hypothesized that the intrauterine manipulation of endometrial cytology resulted in the release of CT from the uterine cavity to the pelvis via the fallopian tubes.
Two factors were postulated to be involved in this process. First, bleeding in the uterine cavity flows into the pelvis through the fallopian tubes. This mechanism is known as the Sampson’s theory of retrograde menstruation [14]. If uterine bleeding is heavy, whether it is menstruation or not, blood in the uterus also flows back into the pelvic cavity. This retrograde flow indicates the potential for intrauterine lesions, including bacteria, to invade the pelvic cavity.
Another factor is the artificial detachment of endometrial cells in endometrial cytology. In this case, the patient presented with significant genital bleeding. Artificial manipulation of endometrial cytology resulted in the detachment of significantly numerous CT-infected endometrial cells, which subsequently migrated into the pelvic cavity. The mechanism by which CT-infected endometrial cells migrate into the pelvic cavity and are transported throughout the abdominal cavity is caused by ascites movement within the abdominal cavity. As a result of gravity, the ascites are stored in the peritoneal recesses (cystic uterine fossa and Douglas fossa). Nevertheless, owing to the intestinal peristalsis and diaphragm’s respiratory movement, a hydrostatic pressure difference is created between the lower and upper abdomen, and ascites is transported from the pelvis to the subhepatic and subdiaphragmatic areas, even in the upright position [15]. The abovementioned mechanisms could cause the bacteria to disseminate into the peritoneal cavity early after the endometrial examination was performed, leading to peritonitis development.
In summary, we experienced a case of a woman with a CT infection of the endometrium who presented with genital bleeding and developed peritonitis after an endometrial cytology examination. Thus, she was diagnosed with peritonitis. A CT NAAT was then performed, but the result was negative. Given that the etiology of peritonitis was unknown, broad-spectrum antimicrobial agents were used but were ineffective. Subsequently, CT serum antibody titers were measured, demonstrating positive results for IgG and IgA. Minocycline hydrochloride was then administered, resulting in the successful treatment of peritonitis.
Learning points
In chronic CT infection of the uterus with atypical genital bleeding, CT NAAT may cause false-negative results. When genital bleeding is present, and the endometrium needs to be examined, acute or chronic infection in the uterus should be ruled out. Examining the endometrium with infection may cause the pathogen to migrate from the uterus to the abdominal cavity. If a patient presents with peritonitis symptoms, a sole focus on a false-negative CT NAAT result may not provide an adequate treatment plan when a CT infection is present. If a woman develops peritonitis of unknown cause, measuring antibody titers to CT, even if the NAAT result is negative, is beneficial.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the patient before completing the case report.
Author Contributions
HM and SiI contributed to the manuscript’s writing. KH and EF reviewed and adjusted the manuscript before submission.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
CT: Chlamydia trachomatis; MRI: magnetic resonance imaging; NAAT: nucleic acid amplification testing; PID: pelvic inflammatory disease
| References | ▴Top |
- Yanagisawa N, Tomiyasu H, Hada T, Kure N, Kobayashi Y, Katamoto T, Sugaya H, et al. Chlamydia trachomatis peritonitis: report of a patient presenting spontaneous regression of ascites. Intern Med. 1992;31(6):835-839.
doi pubmed - Peter NG, Clark LR, Jaeger JR. Fitz-Hugh-Curtis syndrome: a diagnosis to consider in women with right upper quadrant pain. Cleve Clin J Med. 2004;71(3):233-239.
doi pubmed - US Preventive Services Task Force, Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, et al. Screening for chlamydia and gonorrhea: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;326(10):949-956.
doi pubmed - Chlamydial Infections. https://www.cdc.gov/std/treatment-guidelines/chlamydia.htm.
- Madenci H, Ugur C, Demirci T. A rare small intestine injury without free gas image on radiological imaging after blunt abdominal trauma in a child: ileal perforation. J Emerg Med Case Rep. 2020;13(1):31-33.
doi - Guarino J, Hassett JM, Jr., Luchette FA. Small bowel injuries: mechanisms, patterns, and outcome. J Trauma. 1995;39(6):1076-1080.
doi pubmed - Hino Y, Eshima N, Bacal K, Tokumaru O. Age- and sex-related differences in morbidities of sexually transmitted diseases in children. Children (Basel). 2021;8(1):40.
doi pubmed pmc - Garibyan L, Avashia N. Polymerase chain reaction. J Invest Dermatol. 2013;133(3):1-4.
doi pubmed pmc - Liang Y, Jin X, Yuan F, Li Z, Chen S. Comparison of rRNA-based and DNA-based nucleic acid amplifications for detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Ureaplasma urealyticum in urogenital swabs. BMC Infect Dis. 2018;18(1):651.
doi pubmed pmc - Cohen CR, Nosek M, Meier A, Astete SG, Iverson-Cabral S, Mugo NR, Totten PA. Mycoplasma genitalium infection and persistence in a cohort of female sex workers in Nairobi, Kenya. Sex Transm Dis. 2007;34(5):274-279.
doi pubmed - Loj B, Brodowska A, Ciecwiez S, Szydlowska I, Brodowski J, Lokaj M, Starczewski A. The role of serological testing for Chlamydia trachomatis in differential diagnosis of pelvic pain. Ann Agric Environ Med. 2016;23(3):506-510.
doi pubmed - Muntaha MH, Noor NR, Tamadher MH. Assessment of Chlamydia trachomatis infection in symptomatic women by ELISA and evaluate the levels of CRP, C3, C4 and IgA in patients sera. J Pharm Sci Res. 2019;11(3):1131-1135.
doi - den Hartog JE, Ouburg S, Land JA, Lyons JM, Ito JI, Pena AS, Morre SA. Do host genetic traits in the bacterial sensing system play a role in the development of Chlamydia trachomatis-associated tubal pathology in subfertile women? BMC Infect Dis. 2006;6:122.
doi pubmed pmc - Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927;3(2):93-110.43.
pubmed pmc - Solass W, Struller F, Horvath P, Konigsrainer A, Sipos B, Weinreich FJ. Morphology of the peritoneal cavity and pathophysiological consequences. Pleura Peritoneum. 2016;1(4):193-201.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.