| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com |
Case Report
Volume 16, Number 5, May 2025, pages 174-180
Treatment of Invasive Fungal Disease During Therapy for Acute Lymphoblastic Leukemia
Ibrahim Alharbia, d, Amro Nassifb, Yasser B. Hennawic
aDepartment of Pediatrics, Umm Al-Qura University, Makkah, Saudi Arabia
bPediatric Division, Department of Pediatrics, King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia
cOrthopedics Department, King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia
dCorresponding Author: Ibrahim Alharbi, Department of Pediatrics, Umm Al-Qura University, Makkah, Saudi Arabia
Manuscript submitted March 12, 2025, accepted May 6, 2025, published online May 28, 2025
Short title: Treatment of IFD for ALL
doi: https://doi.org/10.14740/jmc5066
| Abstract | ▴Top |
Invasive fungal diseases (IFDs) are one of the leading causes of death in acute leukemia (AL) patients. Because of the possibility of fungal relapse, patients who survive IFDs may have difficulty in completing the whole chemotherapy plan. Our case report presents two cases of IFD with aspergillosis in children with precursor B-cell acute lymphoblastic leukemia (pre-B-ALL). Two 9-year-old female patients were diagnosed with pre-B-ALL that were on the pre-B-ALL protocol: CALL08, Arm-C (high-risk arm), and the supportive therapy. They were both on Arm-C of the CALL08 protocol (high risk based on COG232). Then, the patients experienced severe febrile neutropenia. Patient 1 was during consolidation, and patient 2 was during interim maintenance I. Both experienced prolonged febrile neutropenia. As febrile neutropenia continued for more than 5 days, a fungal workup was conducted, including computed tomography (CT) scans of the sinuses, chest, and abdomen, as well as serum tests for galactomannan and (1→3)-β-D-glucan (BDG). Caspofungin treatment was started. Fungal workup results showed lung and liver nodules in one patient and lungs, liver, and spleen in the other. There were about 4 weeks of severe fevers and neutropenia, despite the use of broad-spectrum antibiotics. A decision was taken to interrupt chemotherapy for both patients. Voriconazole was added to caspofungin. Biopsies confirmed the diagnosis to be severe fungal infection with invasive aspergillosis. After that, high fevers and neutropenia slowly recovered, and a repeated CT scan of abdomen showed good improvement in the lesion’s number and size. After 6 - 8 weeks of interruption, chemotherapy was resumed. We observed that with the implementation of combination antifungal therapy with voriconazole and caspofungin for 6 weeks and then single antifungal therapy (voriconazole orally) for another 6 weeks, both patients recovered and became clinically stable and afebrile. Chemotherapy was on hold till they became better. In conclusion, primary and secondary antifungal prophylaxis are recommended for ALL patients. Chemotherapy discontinuation is decided on an individual basis according to the severity of the fungal infection and disease status.
Keywords: Invasive fungal infection; ALL; Antifungal prophylaxis
| Introduction | ▴Top |
Acute lymphoblastic leukemia (ALL) is considered the most common malignancy in children accounting for 25% of all pediatric cancers [1]. The most prevalent complication of precursor B-cell ALL (pre-B-ALL) is infection. Furthermore, there are also gastrointestinal, neurological, metabolic/endocrine, drug-related hypersensitivity, and severe psychological issues, all resulting either from the disease itself or from chemotherapy complications [2]. Chemotherapy can make these children extremely immunocompromised and vulnerable to infections.
In such immunocompromised patients, opportunistic invasive fungal diseases (IFDs) are the leading cause of morbidity and mortality. Moreover, the presence of molds or yeasts in deep tissues can persist and become very hard to get rid of even with optimal antifungal therapy. In addition, it is not very straightforward sometimes to diagnose these infections. We need to do biopsy or a culture obtained by a sterile procedure to prove the existence of IFD [3]. The most prevalent fungal infection in humans is aspergillosis, which accounts for more than 85% of IFDs [4].
The opportunistic infection known as invasive aspergillosis (IA) is caused by Aspergillus spp. Aspergillus is a saprophytic filamentous fungus that is frequently isolated in the environment [5]. Because of difficulties in performing the invasive procedure to diagnose it, a lot of effort has gone into creating a non-invasive test for the diagnosis of IA, especially techniques to find galactomannan [6]. Aspergillus releases galactomannan, a polysaccharide cell wall component, as it grows [6, 7]. Consequently, for monitoring IA, a double-sandwich enzyme-linked immunosorbent galactomannan assay has been licensed [7]. Additionally, (1→3)-β-D-glucan (BDG) is an appropriate material to screen for IFDs, because it is a part of the fungal cell wall and is not present in bacteria or human cells. The G-test, which measures BDG in serum using a coagulation factor derived from horseshoe crab blood, was created initially in Japan [8].
Hematological malignancies, particularly acute myeloid leukemia (AML), ALL, and allogeneic hematopoietic stem cell transplantation (HSCT) recipients, are associated with IA in patients [3, 4]. Extreme neutropenia brought on by chemotherapy (especially lasting > 7 days; absolute neutrophil count (ANC) 500 cells/mm3) continues to be the single biggest risk factor for IA [9].
Fever unresponsive to broad-spectrum antibiotics in neutropenic patients is a common early symptom that should warrant additional investigation. Coughing or chest pain with or without hemoptysis, both symptoms of pulmonary infarction caused by fungal-induced vascular blockage, have been recorded on rare occasions. The critically ill intensive care unit (ICU) patient with IA, on the other hand, is largely mechanically ventilated and may have decreasing lung function and refractory fever. Progression to disseminated disease is a common consequence that goes unnoticed, especially in individuals who have a severe or advanced underlying condition. Cerebral dispersion can reveal itself late in the form of seizures or other focal neurological symptoms, although it is uncommon to have a primary extrapulmonary organ at presentation [9].
We present two challenging cases of 9 years old patients diagnosed with pre-B-ALL and both cases were complicated with IFD (aspergillosis). Therefore, the chemotherapy was interrupted in both cases.
| Case Reports | ▴Top |
Case 1
Case 1 was a 9-year-old girl with a 2-week history of fevers, pallor, and pancytopenia. The physical examination revealed no abnormalities, except that the patient had pale skin without ecchymosis or petechiae. Complete blood count (CBC) showed 2-cell line pancytopenias as well as peripheral blood blasts. The white blood cell (WBC) level was 6.8 × 109/L. Hemoglobin level was 8.6 g/dL, hematocrit (Hct) volume was 24.8%, platelets level was 86 × 109/L, and total bilirubin was 2 µmol/L. Lactate dehydrogenase (LDH) was 702 U/L and went up to 4,000 U/L with the start of induction. Bone marrow aspiration showed 95% blasts, and the flow cytometry showed pre-B-ALL. Results were consistent with pre-B-ALL and the RUNX1 was positive. The recommended plan for management was the pre-B-ALL protocol: CALL08, Arm-C (high-risk Arm) and the supportive therapy.
She was stable and had no major events during 1.5 years after the pre-B-ALL protocol, and then suddenly she started to experience severe febrile neutropenia. There was a belief that her family gave her herbal treatment, but we could not prove it. This sudden onset of deterioration could not be caused or explained by us. As she initially tolerated well chemotherapy.
Despite empirical antibiotics, the patient was still having more than 5 days febrile neutropenia with negative blood culture. Therefore, a fungal workup including a CT scan and empirical caspofungin was started. However, two questionable lung nodules were found in the CT scan that may be fungal. Accordingly, liposomal amphotericin B was started and caspofungin was discontinued. After about 4 weeks of severe fevers and neutropenia, a decision was made to give her granulocyte colony-stimulating factor (G-CSF) at 5 µg/kg/day subcutaneously (SC). After 3 days, her neutrophil count increased to > 3,000 × 109/L. Interim maintenance I of Arm-C of the CALL08 protocol was started and intravenous (IV) vincristine, intrathecal (IT) methotrexate, IV methotrexate, and L-asparaginase were given. We concluded that IFD is probably due to prolonged febrile neutropenia. Therefore, we made the decision to start G-CSF (Filgrastim) to raise neutrophil count.
Two months later, the patient had severe neutropenia again with fevers and chills. CT of chest, abdomen and sinuses showed numerous nodules in the liver, spleen, and kidneys (Fig. 1). Patient also developed severe hyperbilirubinemia, but transaminases were normal. Total bilirubin increased to 464 µmol/L and conjugated bilirubin was 181 µmol/L and gamma-glutamyl transpeptidase (GGT) was 1,097 U/L.
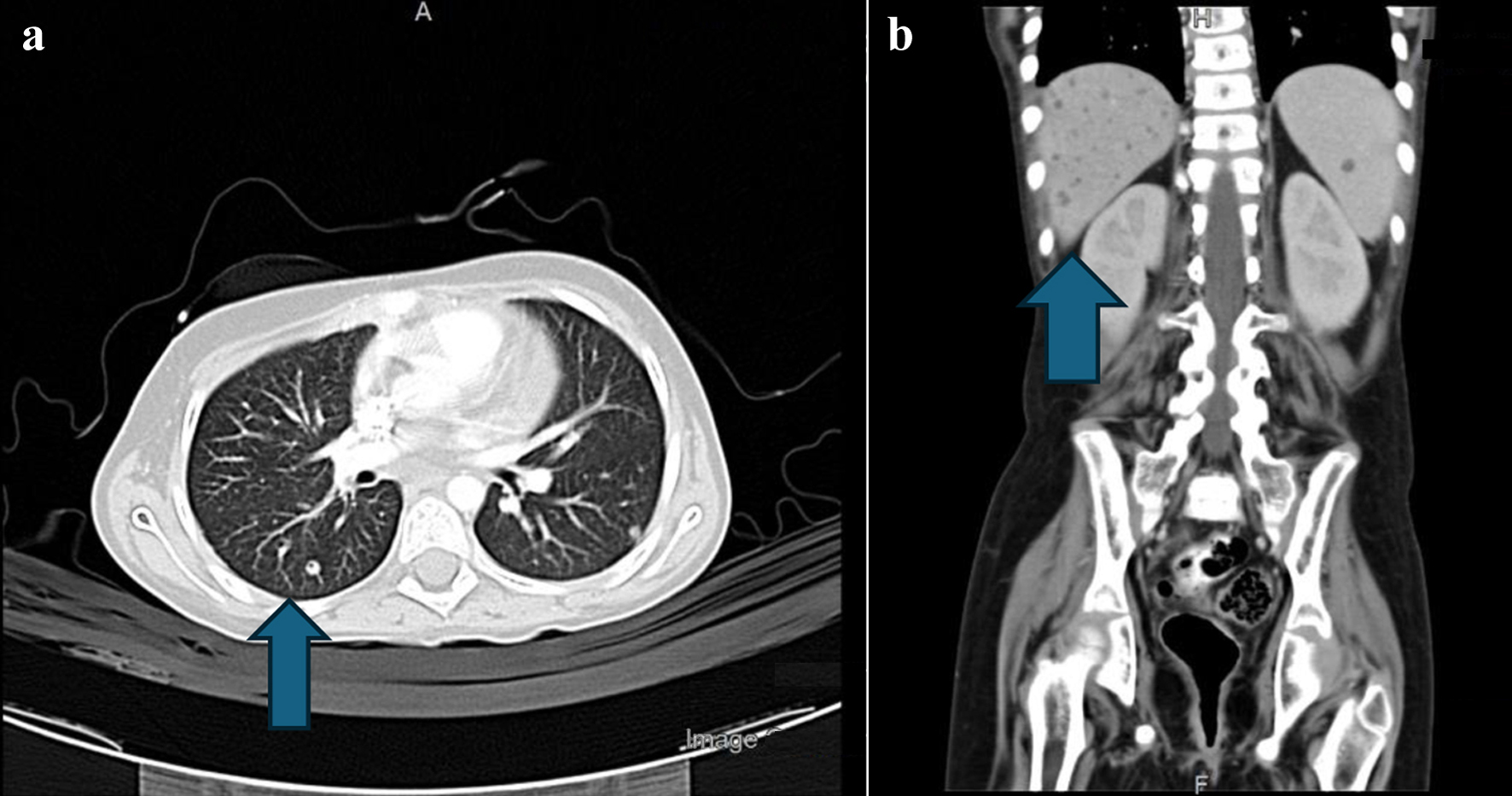 Click for large image | Figure 1. (a) CT scan of the chest. The arrow points to right lung lower lobe, lateral basal segment small, tiny nodule. (b) Abdominal CT with contrast. The arrow shows numerous nodules in the liver, spleen, and kidneys, specifically pointing to right lower liver lobe small nodule. CT: computed tomography. |
The patient started voriconazole and caspofungin combination therapy. Lung nodule biopsy showed hyphae with septate (possible Aspergillus) (Fig. 2). After 9 days, the dose of voriconazole was adjusted to 9 mg/kg q 12 h and the patient continued to improve and nasogastric (NG) feeding was started. After 6 weeks, the patient became stable and afebrile. Hyperbilirubinemia likely secondary to chemotherapy and antifungal was experienced before and during consolidation and resolved. Then the patient is a case of high-risk ALL in remission, on the day 16 of interim maintenance I. Chemotherapy was on hold till she became better.
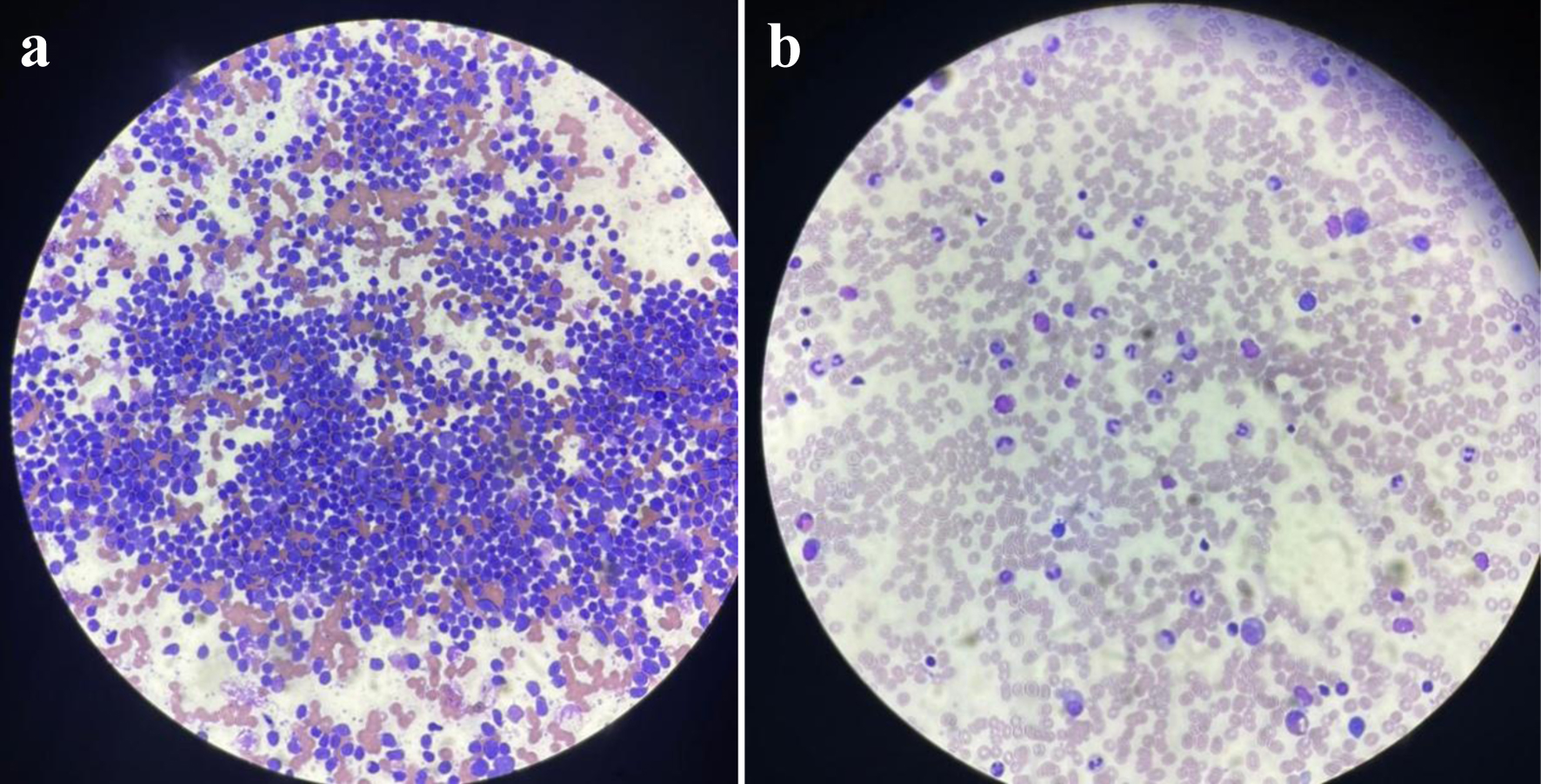 Click for large image | Figure 2. (a) Ultrasound-guided biopsy of lung nodules showing hyphae with septate (possible Aspergillus). (b) Biopsy of lung nodules after fungal treatment. |
Case 2
Case 2 was a 9-year-old girl with newly diagnosed B-ALL. She had pallor, petechiae, and ecchymosis as presenting complaints. On examination, there was pallor, scattered petechiae, mild hepatomegaly, and palpable spleen tip without significant lymphadenopathy. Investigations were done and the WBC count was 12.6 × 109/L, the Hb was 6.5 g/dL, and the platelet count was 94 × 109/L.
Immunophenotyping was done on the provided bone marrow specimen by flow cytometry.
It showed an abnormal B-cell population in the CD45 dim gate (about 83% of all the cells analyzed). These B cells had intermediate nuclear size (based on forward-scatter signal) and showed expression of CD10, CD19, CD38, HLA-DR, CD79a, cTdT, and CD22.
They also showed aberrant expression of CD13 and CD33 which can be used for monitoring minimal residual disease (MRD). The blast population showed partial expression of CD24 and cIgM.
They were negative for CD34, CD20, CD117, MPO, CD3, CD5, kappa, and lambda.
Residual lymphocytes constituted about 15% of the analyzed cells. The overall immunophenotype results were most consistent with B-ALL.
A CT scan of chest showed new tiny pulmonary nodules, while an abdominal CT scan showed bilateral kidneys with scattered focal hypodensities, with the largest being seen in the left kidney (Fig. 3). The diagnosis was a case of B-ALL and initially she was assigned to standard risk chemotherapy protocol (Arm-A which is COG-331).
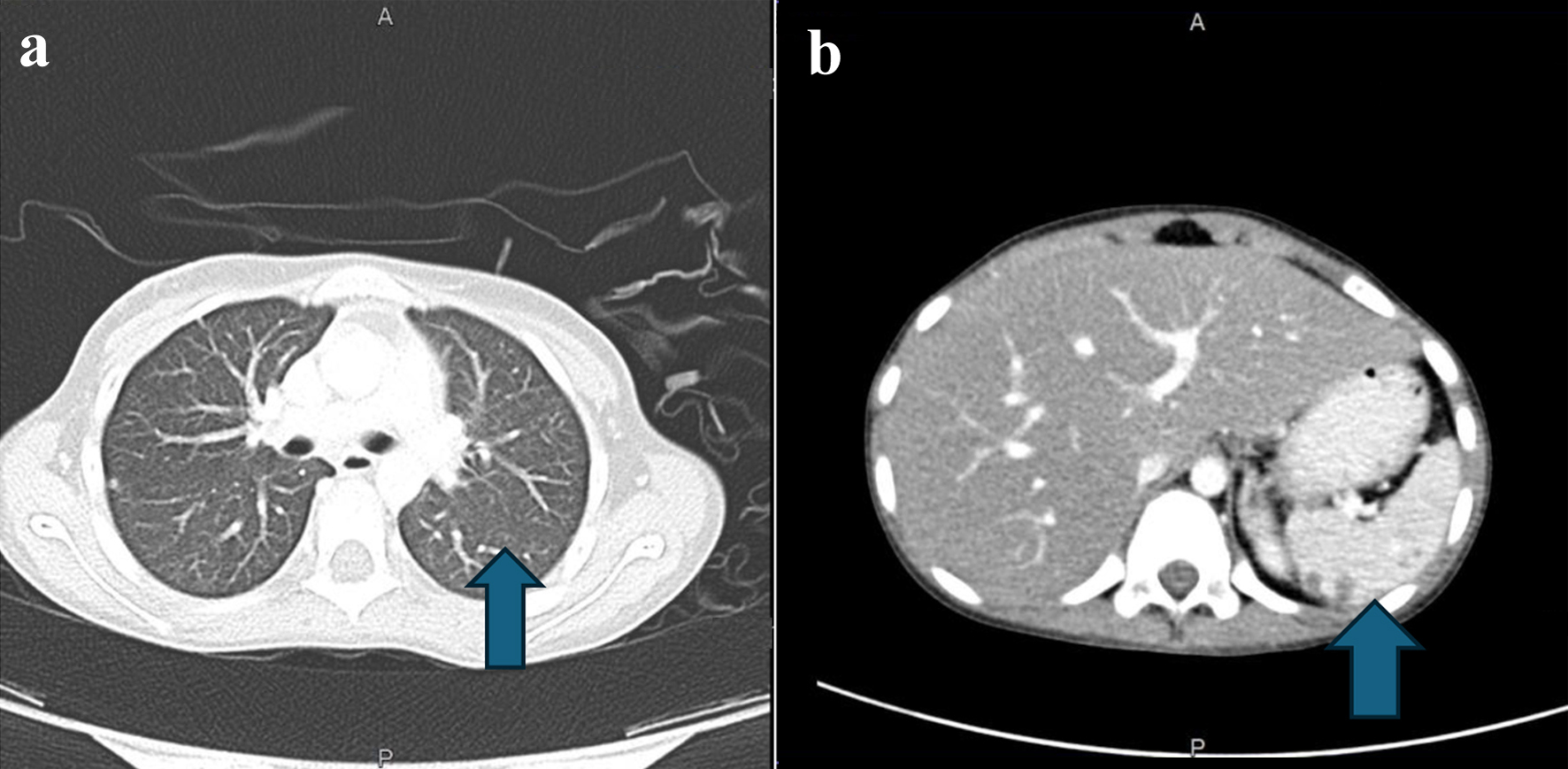 Click for large image | Figure 3. (a) CT scan of the chest. The arrow points to left lung lower lobe, lateral basal segment small, tiny nodule. (b) Abdominal CT with contrast. The arrow points to multiple nodules in the spleen. CT: computed tomography. |
She was started on voriconazole at an induction dose of 9 mg/kg for 1 day, followed by a maintenance dose of 8 mg/kg given twice per day. Reassessment showed mixed response with regressions and reappearance of some nodule, therefore, the dose was increased to 14 mg/kg given twice per day. However, she experienced elevated liver enzymes which could be attributed to the use of medications. Liposomal amphotericin B was added at 5 mg/kg IV given once per day for 1 month; at that time, she had resistant electrolytes imbalance in form of low potassium. This is hypokalemia most likely due to the use of medication. As a result, she was shifted to oral posaconazole 100 mg given twice per day orally, but she continued to have hypokalemia. Infectious Disease and Nephrology teams consultations were done and they recommended to reduce the dose of posaconazole to 100 mg given once per day orally which showed marked improvement of hypokalemia and fungal assessment showed static nodules. Plan was to continue chemotherapy and antifungal until the end of maintenance chemotherapy.
Now she is on chemotherapy (Arm-C, delayed intensification #1 week 29), posaconazole 100 mg given once per day orally and bactrim 50 mg given twice per day orally 2 days weekly.
| Discussion | ▴Top |
In these two cases, both patients were diagnosed with pre-B-ALL and were complicated with IFD (aspergillosis).
The risk of IFDs is highly unpredictable due to the variability of severity of disease in children with ALL. It seems to be the result of interactions between three factors: immunosuppression, organ failure, and exposure to opportunistic fungi [10]. Immunosuppression is caused by corticosteroids and chemotherapy, leading to dysfunction in cell-mediated, humoral, and phagocytic responses, which results in a predisposition to IFDs [11].
It was discovered that exposure to opportunistic fungi also increases the incidence of IFDs. It has been well known that Aspergillus species colonies the airways of many individuals [12]. In adult patients with newly diagnosed AML, Italian researchers found a substantial link between pre-hospitalization exposure to fungus sources and the development of IFDs within 30 days after the first session of chemotherapy [13]. Inhalation of fungal spores is critical in the development of IFDs [13]. IFDs are a leading cause of mortality and morbidly in pediatric patients with AL. It affects 5-15% of pediatric patients with AL [14]. The majority of these cases are ALL. IA patients are reported to have a death rate of 28-42% three months following diagnosis [15].
Our cases showed numerous nodules of severe fungal infection with IA in the liver as well as in the spleen and kidneys on repeated fungal workup (CT of abdomen, chest, and sinuses). According to a retrospective review, nodules were the most common diagnostic radiological finding in 48 (34.6%) out of 139 pediatric IA cases [16].
Both patients initially started on liposomal amphotericin, and then continued on a combination of caspofungin and voriconazole therapy. Because of its excellent efficacy, voriconazole belongs to a class of antifungal called triazoles. It acts by slowing fungal growth. It is used to treat infections with many fungal infections including aspergillosis, candidiasis, coccidioidomycosis, histoplasmosis, penicilliosis, and infections by Scedosporium or Fusarium. It is also used as a prophylaxis to prevent IFDs. International guidelines recommend administration of voriconazole as pyrophytic agent against IFDs [17, 18]. In addition, voriconazole is the drug of choice for aspergillosis [17, 19]. About 86% of patients with IFD who received subsequent antifungal prophylaxis would have had reduced fungal reactivation risk [18]. Antifungal prophylaxis was also advised for AML patients receiving induction chemotherapy [20]. Caspofungin is a drug that belongs to Echinocandins class. It has a broad-spectrum fungicidal activity against many fungal infections including Candida and fungistatic activity against Aspergillus species [21]. It is specifically used to treat invasive Candidiasis, like esophageal Candidiasis, and IA. It may be explored for prophylaxis against IFD in children, adolescents, and young adults with AL, according to Fisher et al (2019) [22]. In a randomized trial of adolescents and adults with AML, the risk of IFDs was reduced with caspofungin prophylaxis and that was equivalent to posaconazole prophylaxis [21]. Other trials found that voriconazole, amphotericin B, caspofungin, or posaconazole, alone or in combination, were effective in treating IFDs in ALL individuals [23, 24].
The chemotherapy treatment protocols of both of our cases were interrupted for more than 4 weeks. To avoid risk of relapse, patients needed to have some form of mild chemotherapy in order to keep them both in a continuous remission. Based on most recent evidence, they both received IV vincristine every 2 - 3 weeks as well as IT methotrexate every 3 - 4 weeks.
Therefore, chemotherapy schedule in both of our cases was interrupted. According to a recent study, patients with IFD, despite initially surviving the infections, have considerable risk of persistent infection unless alterations are carried out in their chemotherapeutic schedule. Patients who are receiving chemotherapy and had IFD will be at risk of serious life-threatening consequences unless chemotherapy is interrupted and neutrophil count is allowed to normalize for a long period of time [18]. There is a high probability of persistent/recurrent fungal infections which is believed to be around 30% in patients who survive first IFD if not treated well. In addition, these patients may have difficulty in completing the whole chemotherapy protocol [25]. Those patients frequently have delayed and/or reduction of their chemotherapy doses. Reduction of chemotherapy doses continues until IFDs is controlled [26]. According to the Even et al (2011) study which showed that despite an initial satisfactory outcome, the appearance of IFD during AL treatment has led to a change in the chemotherapy protocol, either delaying the following cycle, changing the drug, or reducing the dosage [18]. The principle behind reducing the chemotherapy intensity is to allow full and prolonged neutrophil count recovery.
Our cases experienced about 4 weeks of severe fevers and neutropenia. We had to interrupt their chemotherapy for a prolonged period of time. Regarding which AL is more likely to be associated with IFD, researchers from a single Taiwanese hospital found in 2011 that invasive fungal sinusitis (IFS) was more prevalent in AML patients with persistent neutropenia throughout a 15-year period than with ALL [27]. When considering whether the duration of neutropenia is associated with the risk of IDF, Lien et al (2018) found a direct link between the length of neutropenia (more than 30 days) and the significant risk of IFD [20]. When considering if there are other factors that can contribute to the risk of IFD in addition to prolonged neutropenia, a retrospective investigation by Nicolato et al found that prolonged neutropenia, relapse of underlying illnesses, extended hospitalization before the first fever, and allogeneic HSCT were all risk factors for IFD in ALL. These risk factors have also been seen in our case [25]. The generation of normal neutrophils decreases as leukemia cells proliferate in the bone marrow [17]. According to another recent study, the majority of our patients (88%) were severely neutropenic at the time of diagnosis of IFD and the overall median duration of neutropenia was longer than 10 days [27].
Previous research has shown that fast and effective antifungal medication combined with sensible treatment modalities can reduce the risk of death in children with ALL and IFD. If chemotherapy is held and interrupted for neutrophil count full recovery for a sufficient period of time that allow control of IFD, then chemotherapy can be restarted after a few weeks of interruption [27]. The perfect exact timing to resume full chemotherapy doses and schedules in these patients is unknown, although we believe the exact time is most likely when fungal lesions are resolved and disappeared on further imaging evaluation [23].
What is the optimal duration of time of chemotherapy interruption? In our cases, interruption ranged from 4 to 12 weeks. Previous data have produced similar results. According to Tufekci et al (2015), the median time to stop chemotherapy was 27 days, and chemotherapy was safely restarted in 50% of the patients before 4 weeks duration. IFD was not reactivated in any of those patients [27]. In a retrospective assessment of hematological malignancies, Tufekci et al (2015) found 61 adult instances with IFD and discovered a median period of 27 days for chemotherapy termination (range: 17 - 45 days) was appropriate [27].
Conclusion
Primary prophylaxis, empirical or preemptive antifungal medication, and secondary prophylaxis are all options for treating patients. Chemotherapy timing is usually decided on an individual basis, depending on the severity of the fungal infection and the status of the main disease.
Learning points
The presence of an IFD in a pediatric patient with ALL is a major challenge. It is difficult, most likely related to chemotherapy. Risk factors are intensity chemotherapy and neutropenia. Multidisciplinary teams such as pediatric hematology-oncology, pediatric infectious disease, and allergy immunology can improve outcomes and manage this condition more effectively.
Our treatment strategy now was on the rationale that, in order to avoid treatment interruptions, we will implement maximum measures to address infection control and isolation policies. In addition, we would like to keep our patients on fungal prophylaxis especially during first few phases of therapy.
Acknowledgments
We would like to thank the ethical committee team in King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia.
Financial Disclosure
All authors have declared that they have no financial relationships at present or within the previous 3 years with any organizations that might have an interest in the submitted work.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained or waived by the participant in this study.
Author Contributions
All authors have reviewed the final version to be published and agreed to be accountable for all aspects of the work. Interpretation of data: Ibrahim Alharbi. Drafting of the manuscript: Yasser B. Hennawi, Amro Nassif. Critical review of the manuscript for important intellectual content: Ibrahim Alharbi, Yasser B. Hennawi, and Amro Nassif. Supervision: Ibrahim Alharbi.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381(9881):1943-1955.
doi pubmed pmc - Ozturk AP, Koc B, Zulfikar B. Acute complications and survival analysis of childhood acute lymphoblastic leukemia: a 15-year experience. Clin Lymphoma Myeloma Leuk. 2021;21(1):e39-e47.
doi pubmed - Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, Denning DW, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34(1):7-14.
doi pubmed - Alastruey-Izquierdo A, Mellado E, Pelaez T, Peman J, Zapico S, Alvarez M, Rodriguez-Tudela JL, et al. Population-based survey of filamentous fungi and antifungal resistance in Spain (FILPOP Study). Antimicrob Agents Chemother. 2013;57(7):3380-3387.
doi pubmed pmc - Hites M, Goicoechea Turcott EW, Taccone FS. The role of galactomannan testing to diagnose invasive pulmonary aspergillosis in critically ill patients. Ann Transl Med. 2016;4(18):353.
doi pubmed pmc - Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis. 2006;42(10):1417-1427.
doi pubmed - Leeflang MM, Debets-Ossenkopp YJ, Wang J, Visser CE, Scholten RJ, Hooft L, Bijlmer HA, et al. Galactomannan detection for invasive aspergillosis in immunocompromised patients. Cochrane Database Syst Rev. 2015;2015(12):CD007394.
doi pubmed pmc - Tamura H, Arimoto Y, Tanaka S, Yoshida M, Obayashi T, Kawai T. Automated kinetic assay for endotoxin and (1—>3)-beta-D-glucan in human blood. Clin Chim Acta. 1994;226(1):109-112.
doi pubmed - Schmiedel Y, Zimmerli S. Common invasive fungal diseases: an overview of invasive candidiasis, aspergillosis, cryptococcosis, and Pneumocystis pneumonia. Swiss Med Wkly. 2016;146:w14281.
doi pubmed - Anaissie EJ, McGinnis MR, Pfaller MA. Clinical mycology. 2nd ed. Philadelphia: Churchill Livingstone/Elsevier; 2009.
- Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(3):327-360.
doi pubmed - Nucci M, Anaissie E. How we treat invasive fungal diseases in patients with acute leukemia: the importance of an individualized approach. Blood. 2014;124(26):3858-3869.
doi pubmed - Haleem Khan AA, Mohan Karuppayil S. Fungal pollution of indoor environments and its management. Saudi J Biol Sci. 2012;19(4):405-426.
doi pubmed pmc - Pagano L, Caira M, Candoni A, Offidani M, Martino B, Specchia G, Pastore D, et al. Invasive aspergillosis in patients with acute myeloid leukemia: a SEIFEM-2008 registry study. Haematologica. 2010;95(4):644-650.
doi pubmed pmc - Reboli AC, Rotstein C, Pappas PG, Chapman SW, Kett DH, Kumar D, Betts R, et al. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med. 2007;356(24):2472-2482.
doi pubmed - Burgos A, Zaoutis TE, Dvorak CC, Hoffman JA, Knapp KM, Nania JJ, Prasad P, et al. Pediatric invasive aspergillosis: a multicenter retrospective analysis of 139 contemporary cases. Pediatrics. 2008;121(5):e1286-e1294.
doi pubmed - Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, Kern WV, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347(6):408-415.
doi pubmed - Even C, Bastuji-Garin S, Hicheri Y, Pautas C, Botterel F, Maury S, Cabanne L, et al. Impact of invasive fungal disease on the chemotherapy schedule and event-free survival in acute leukemia patients who survived fungal disease: a case-control study. Haematologica. 2011;96(2):337-341.
doi pubmed pmc - Cordonnier C, Rovira M, Maertens J, Olavarria E, Faucher C, Bilger K, Pigneux A, et al. Voriconazole for secondary prophylaxis of invasive fungal infections in allogeneic stem cell transplant recipients: results of the VOSIFI study. Haematologica. 2010;95(10):1762-1768.
doi pubmed pmc - Lien MY, Chou CH, Lin CC, Bai LY, Chiu CF, Yeh SP, Ho MW. Epidemiology and risk factors for invasive fungal infections during induction chemotherapy for newly diagnosed acute myeloid leukemia: A retrospective cohort study. PLoS One. 2018;13(6):e0197851.
doi pubmed pmc - Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356(4):348-359.
doi pubmed - Fisher BT, Zaoutis T, Dvorak CC, Nieder M, Zerr D, Wingard JR, Callahan C, et al. Effect of caspofungin vs fluconazole prophylaxis on invasive fungal disease among children and young adults with acute myeloid leukemia: a randomized clinical trial. JAMA. 2019;322(17):1673-1681.
doi pubmed pmc - Katragkou A, Roilides E. Best practice in treating infants and children with proven, probable or suspected invasive fungal infections. Curr Opin Infect Dis. 2011;24(3):225-229.
doi pubmed - Maertens J, Groll AH, Cordonnier C, de la Camara R, Roilides E, Marchetti O. Treatment and timing in invasive mould disease. J Antimicrob Chemother. 2011;66(Suppl 1):i37-i43.
doi pubmed - Nicolato A, Nouer SA, Garnica M, Portugal R, Maiolino A, Nucci M. Invasive fungal diseases in patients with acute lymphoid leukemia. Leuk Lymphoma. 2016;57(9):2084-2089.
doi pubmed - Sipsas NV, Kontoyiannis DP. Clinical issues regarding relapsing aspergillosis and the efficacy of secondary antifungal prophylaxis in patients with hematological malignancies. Clin Infect Dis. 2006;42(11):1584-1591.
doi pubmed - Tufekci O, Yilmaz Bengoa S, Demir Yenigurbuz F, Simsek E, Karapinar TH, Irken G, Oren H. Management of invasive fungal infections in pediatric acute leukemia and the appropriate time for restarting chemotherapy. Turk J Haematol. 2015;32(4):329-337.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.