| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com/ |
Case Report
Volume 15, Number 12, December 2024, pages 371-375
Gastric Schwannoma: A Rare Cause of Gastric Bleeding
Daniela Pato Paisa, b , Sara Andradea, Ines Bertao Colacoa, Margarida Luisa, Nuno Azenhaa, Ana Couceiroa, Jose Valente Cecilioa
aDepartment of Surgery, Hospital Distrital da Figueira da Foz, Rua do Hospital 3094-001, Figueira da Foz, Portugal
bCorresponding Author: Daniela Pato Pais, Department of Surgery, Hospital Distrital da Figueira da Foz, Rua do Hospital 3094-001, Figueira da Foz, Portugal
Manuscript submitted August 18, 2024, accepted October 14, 2024, published online October 25, 2024
Short title: Gastric Schwannoma as a Rare Cause of Bleeding
doi: https://doi.org/10.14740/jmc4312
| Abstract | ▴Top |
Gastric schwannomas and gastrointestinal stromal tumors (GISTs) are two types of mesenchymal tumors, which represent a group of rare tumors of the gastrointestinal tract. The differential diagnosis between these two tumors is difficult given their very similar appearance and clinical features. The authors present a case of a 63-year-old man with melena and epigastric pain. An upper digestive endoscopy was performed, revealing an ulcerated gastric subepithelial lesion suspected to be a GIST. Further imaging with a computed tomography (CT) scan revealed a well-defined hypodense solid nodular mass, with homogeneous enhancement, measuring 22 × 18 mm, on the anterior wall of the transition between the body and gastric antrum, situated within the submucosal layer. The patient subsequently underwent a laparoscopic atypical gastrectomy, which proceeded without complications. The pathological examination of the excised lesion confirmed it to be a gastric schwannoma, with complete excision. This case report illustrates a rare cause of gastrointestinal bleeding, that requires immediate action, and en bloc resection is usually curative. Given the excellent prognosis after complete resection, a correct diagnosis is essential.
Keywords: Abdominal pain; Gastric lesion; Gastrointestinal oncology; Schwannoma; Laparoscopic surgery
| Introduction | ▴Top |
Gastric schwannomas are rare tumors, originating from Schwann cells [1], representing only 0.2% of all gastric neoplasms [2]. Gastric tumors are classified as epithelial and non-epithelial [3]. Epithelial tumors originate in the mucosa, and non-epithelial tumors originate more deeply in the submucosa, muscularis propria or serosa [3]. The group of non-epithelial or mesenchymal tumors includes gastrointestinal stromal tumors (GISTs), leiomyomas, leiomyosarcomas and schwannomas [3].
Schwannomas are benign neurogenic tumors, most often located in the head and neck [1]. This type of tumors generally accompany the axons of peripheral nerves, but can theoretically affect the paths of any peripheral nerve [4]. When located in the gastrointestinal tract, these tumors derive from the Auerbach nerve plexus [5]. The gastric location is rare, first described in 1988 [6], most frequently located in the gastric body, followed by the antrum and fundus [7].
They are more common in women [3, 8] with a 2:1 ratio [5], and occur most frequently between the fourth and sixth decade of life [8]. Malignant transformation is rare [9, 10]. Most are asymptomatic [4, 5], and the most common clinical presentation is upper gastrointestinal bleeding with abdominal pain [4, 11]. This happens when submucosal growth compromises the vascularization of the underlying mucosa with secondary ischemia [4]. Less frequently, they can present with dyspepsia and palpable mass [11, 12].
The authors present a case involving a gastric tumor, initially diagnosed as a GIST, which was excised. It was later identified as a gastric schwannoma after pathological and immunohistochemical analysis. A retrospective examination of the case was carried out and presented, based on clinical and operative findings, as well as patient follow-up.
This case highlights the importance of the differential diagnosis of submucosal gastric tumors and the presence of rare entities such as gastric schwannoma. The purpose of this study, in addition to analyzing the present clinical case, also aims to carry out a literary review on this topic, addressing the clinical, histopathological characteristics, treatment and prognosis of this entity, promoting a better understanding of this type of tumor.
| Case Report | ▴Top |
Investigations
A 63-year-old man was referred to the Emergency Department due to melena, accompanied by epigastric pain over several days. Apart from this, the patient denied any other symptoms, such as anorexia, nausea, vomiting, dysphagia or weight loss.
His medical history included high blood pressure and dyslipidemia, without further illnesses reported. His regular medication regimen included atorvastatin 20 mg once daily, lisinopril 20 mg once daily and amlodipine 5 mg once daily. He had no history of previous surgeries.
At the time of arrival, the patient’s vital signs were as follows: blood pressure 120/70 mm Hg, heart rate 77/min, respiratory rate 15/min, temperature 37.0 °C, and peripheral oxygen saturation was 94%. On physical examination, the abdominal examination revealed no pain, tenderness or palpable masses. A rectal exam was performed, which confirmed the presence of melena.
Diagnosis
To determine the origin of the bleeding, he was referred for an upper digestive endoscopy that revealed a subepithelial lesion measuring approximately 30 mm in diameter, extensively ulcerated, with hemorrhagic friable edges, located at the greater gastric curvature, at the transition between the body and the antrum (Fig. 1). Multiple punch biopsies were performed, but they did not yield representative samples of the lesion. A thoracic, abdominal, and pelvic computed tomography (CT) was performed, revealing a well-defined hypodense solid nodularity, with homogeneous and frank enhancement, measuring 22 × 18 mm, which suggested a submucosal GIST, without any other abnormalities such as metastases (Fig. 2).
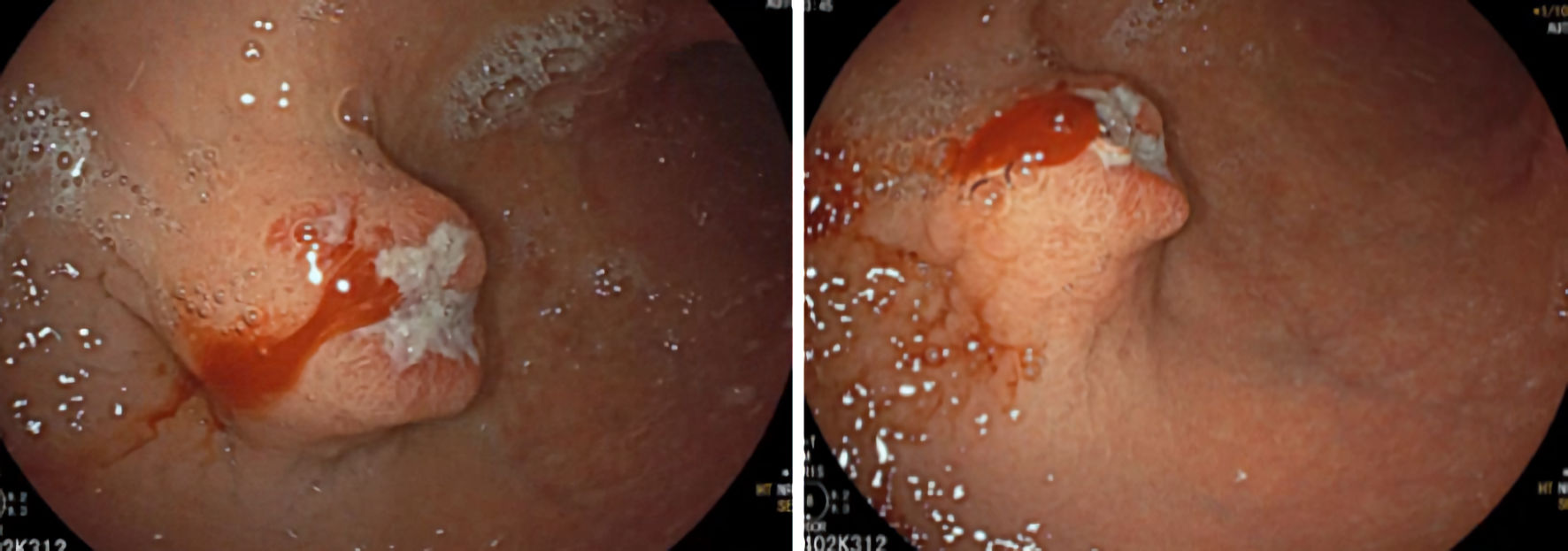 Click for large image | Figure 1. Upper gastrointestinal endoscopy showing subepithelial lesion located on the anterior surface/greater curvature of the middle/distal body, ulcerated. |
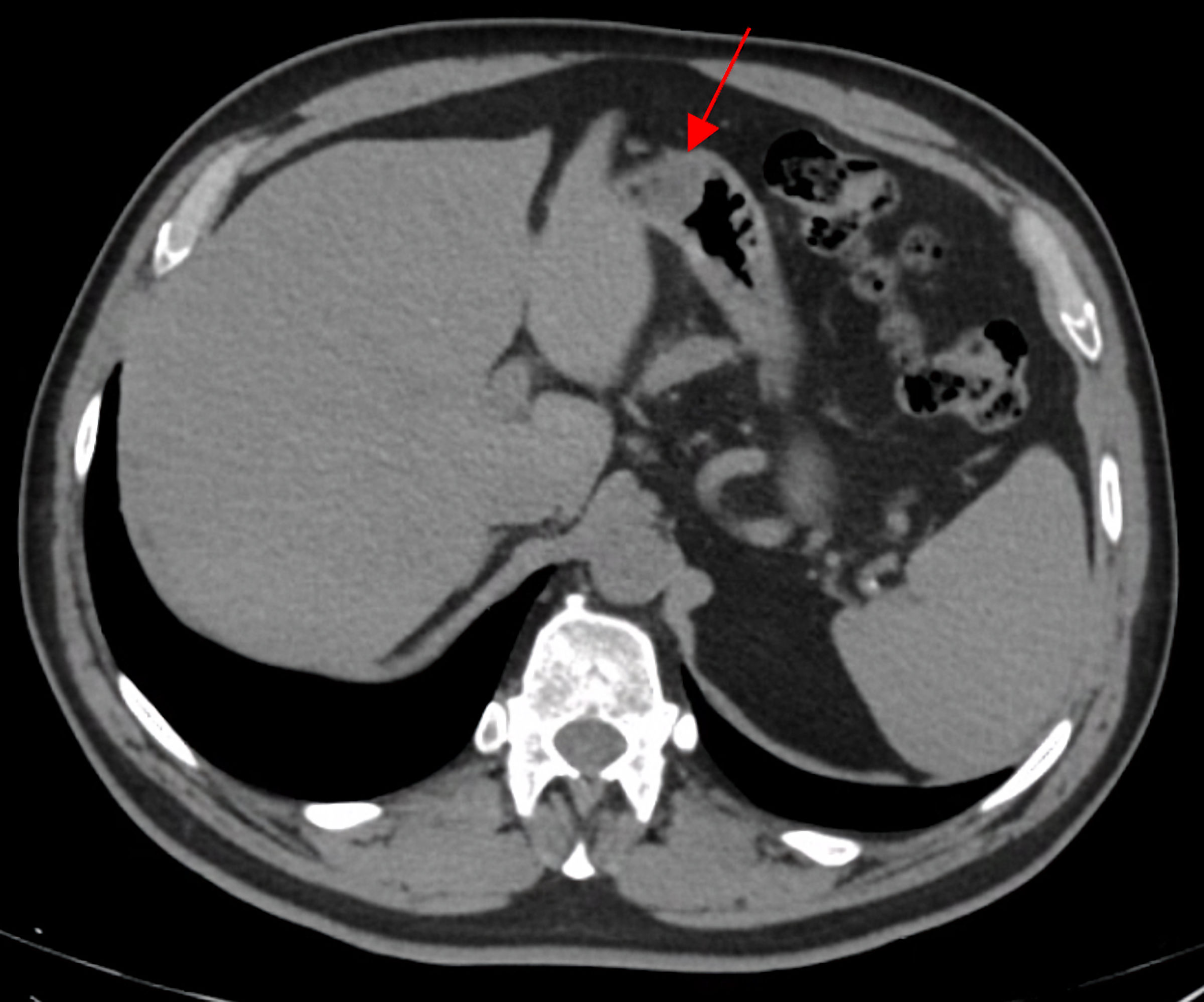 Click for large image | Figure 2. Abdominal and pelvic tomography scan. In a submucosal position, on the anterior wall of the transition between the body and the gastric antrum, a well-defined hypodense solid nodularity, with homogeneous and frank enhancement, measuring 22 × 18 mm (red arrow) was observed. |
Treatment
Considering the imaging and endoscopic findings suggestive of GIST, as well as the patient’s symptoms, surgical treatment was decided without representative biopsies of the lesion. The patient was proposed for excision, having undergone laparoscopic atypical gastrectomy. In the operating room, nodular lesion was observed in the greater gastric curvature, at the gastric antrum. As the lesion was easily identified by laparoscopy, there was no need to perform an upper gastrointestinal endoscopy intraoperatively. A stapled wedge resection was performed with careful preservation of the gastric lumen.
The immediate postoperative period was uneventful, and he was discharged on the fourth postoperative day. Pathological examination of the excised specimen revealed a submucosal nodular formation, centered on the muscularis mucosae, with involvement of the muscularis propria, supported by a myxoid stroma and composed of a proliferation of spindle cells, with expression for CD34 and SOX10, without immunostaining for S-100 protein, actin Ml, desmin, STAT6, DOG1 or c-kit, compatible with schwannoma, with complete excision (Figs. 3-5).
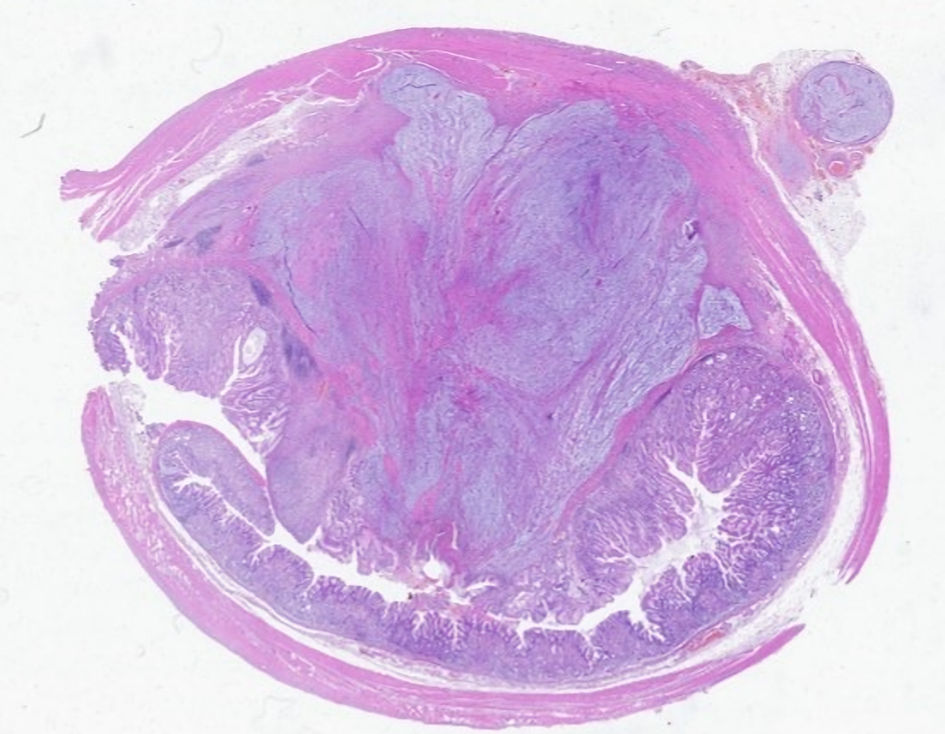 Click for large image | Figure 3. Microscopic analysis. The lesion developed in dependence on the gastric wall. |
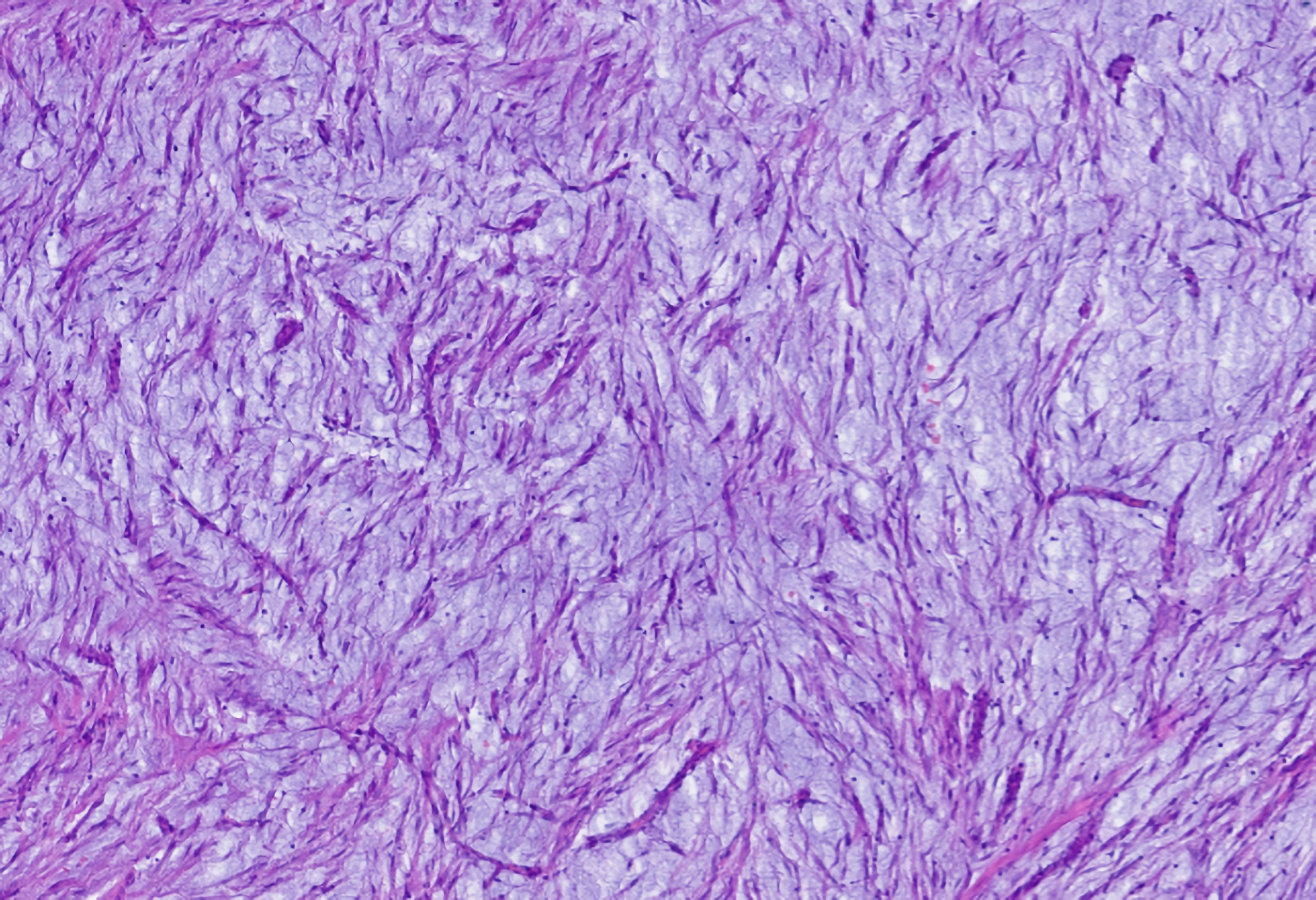 Click for large image | Figure 4. Microscopic analysis showing proliferation of spindle cells on a background with a myxoid appearance. |
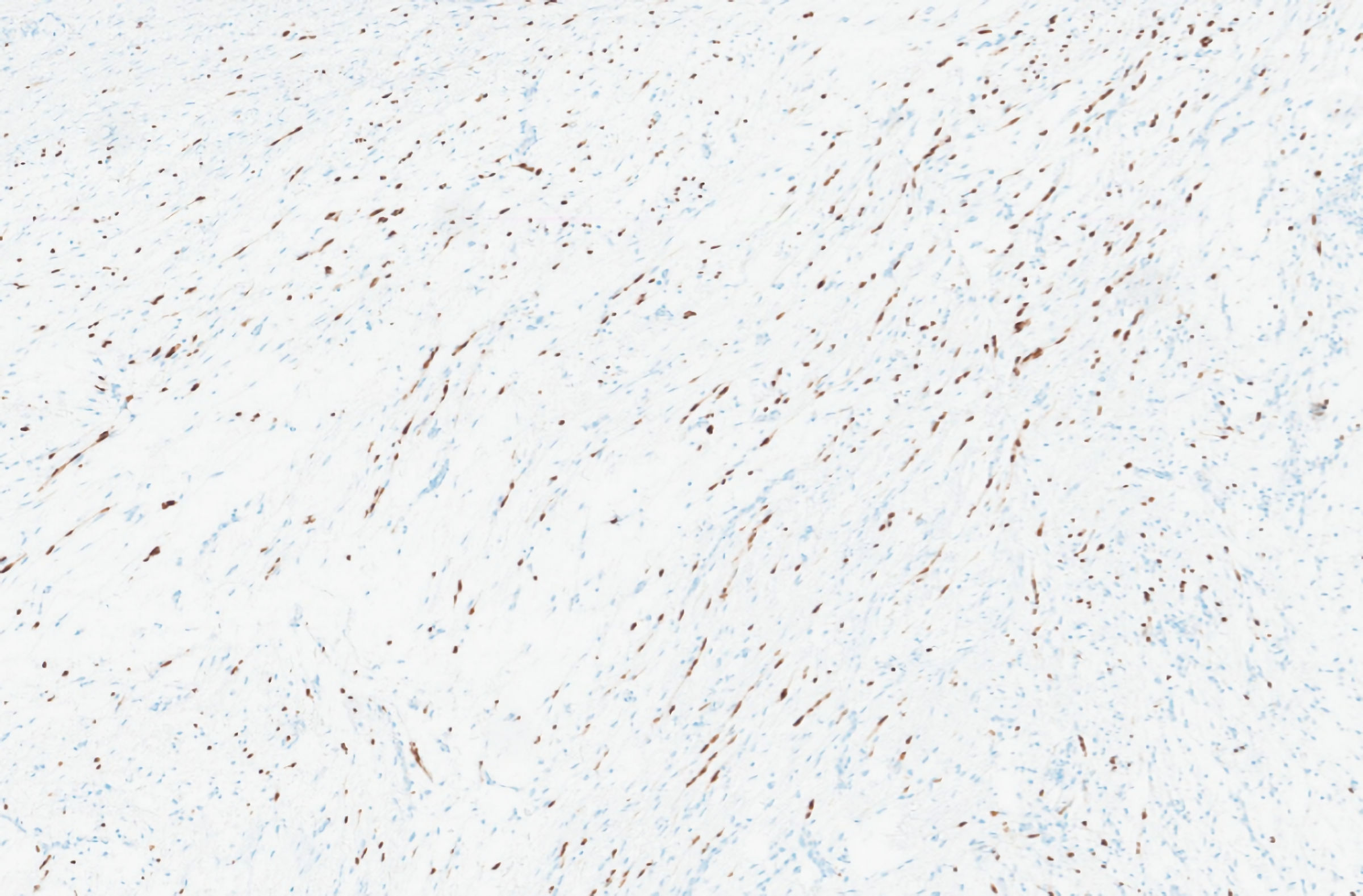 Click for large image | Figure 5. Immunohistochemical analysis showing SOX10 immunoreactive cells. |
Follow-up and outcomes
Postoperative recovery was uneventful. He was observed at 1 and 3 months postoperatively, with complete resolution of gastric symptoms. The histological result of the surgical specimen was discussed with the patient, and its prognosis after complete resection of the tumor was explained.
The patient remains asymptomatic and continues regular follow-up at present to monitor for possible recurrence.
| Discussion | ▴Top |
The authors describe a case of gastric bleeding caused by a schwannoma, initially suspected to be a GIST, a much more common tumor with a significantly different prognosis. The definitive diagnosis was made only after pathological and immunohistochemical analysis.
The most relevant differential diagnosis of a subepithelial gastric lesion is GIST [4], as it is the most common mesenchymal tumor [5]. Both GISTs and gastric schwannomas have very similar appearance and clinical features, with a predominance in similar age groups. Due to some coinciding histological characteristics, some reports prior to modern immunohistochemical techniques may have confused these two types of tumors [8]. On upper gastrointestinal endoscopy, gastric schwannoma appears as an elevated submucosal lesion with a smooth overlying mucosa or occasional ulceration, similar to gastric GIST [3]. Despite these similarities, their treatment differs, as GIST must be classified according to its risk of local recurrence and metastasis, sometimes requiring adjuvant therapy with tyrosine kinase inhibitors in high-risk cases.
Some imaging tests such as CT, magnetic resonance imaging (MRI), and endoscopic ultrasonography (EUS) can help distinguish these two entities [10], although none of the characteristics detected in these studies are pathognomonic [13]. Given the rarity of this diagnosis, there are limited published data on its imaging characteristics [4]. On CT, GISTs most frequently present malignant characteristics, such as hypervascularization, greater volume, necrosis and cystic transformation. Generally, schwannomas tend to be homogeneous and smaller in size, with very rare cystic transformation, necrosis and perilesional adenopathies [14]. The MRI characteristics of these tumors are hypo- or isointense on T1-weighted images and iso- or hyperintense on T2-weighted images [9]. EUS usually shows homogenous, sometimes exophytic, submucosal tumors [15]. It helps delimit the depth of the tumor and also guides the needle biopsy if necessary [3]. However, it is difficult to distinguish between schwannoma, GIST and leiomyoma, as all of these tumors originate in the same layers of the gastric wall [1].
Despite the imaging characteristics, the definitive diagnosis is histopathological [10]. In this case, multiple biopsies were attempted to obtain tissue samples of the lesion, but they did not yield representative results for a conclusive diagnosis, complicating the diagnostic process. A correct preoperative diagnosis is advantageous, as it provides information about the prognosis to the patient and allows for more informed decision-making.
En bloc resection with negative margins is generally the recommended treatment [3], either open or laparoscopic, without the need for lymphadenectomy [10]. Due to its generally benign nature, lymph node metastasis occurs very rarely [10]. Surgical options include wedge resection, local resection, and subtotal or total resection, although wedge resection is the most frequently performed procedure [16].
With the evolution of minimally surgical techniques, several studies have demonstrated several advantages of these techniques, such as smaller incisions, lower risk of complex incisional hernias, less bowel manipulation which leads to a lower incidence of postoperative ileus, reduced postoperative pain and analgesia requirements and a faster functional recovery. Therefore, the preference for open surgery has gradually diminished due to the advantages of minimally invasive techniques [16].
In addition to surgical techniques, endoscopic procedures prove to be safe and effective techniques such as endoscopic submucosal dissection, endoscopic full-thickness resection, and ligation-assisted endoscopic enucleation [10, 12]. However, not all the tumors are suitable for endoscopic excision. There are a few unfavorable characteristics, such as the large size of the tumor (> 5 cm), extraluminal growth, central ulceration, and difficult locations of the lesion (gastric incisura or upper gastric body of lesser curvature), which advise against endoscopic resection [12].
In these cases, interaction with gastroenterologists is crucial. In addition to allowing biopsies to be obtained and thus histological diagnosis, they also help characterize and define the correct location of this type of lesion, helping the surgeon decide the most accurate approach. Furthermore, small tumors may not be easily recognized when laparoscopic surgery is performed. In these cases, this interaction of these two medical specialties becomes fundamental. By performing intraoperative upper digestive endoscopy, it is possible to locate and delimit the lesion, ensuring the excision of the entire lesion. Through this additional examination, an excessively wide excision or even an atypical gastrectomy without the lesion identified can be avoided.
Histological analysis generally shows spindle cells and Schwann cells (Antoni A) [12], organized in a microtrabecular-microfascicular pattern (Verocay bodies) [8, 12], with lymphoid infiltrates at the periphery [1] and hypocellular areas with more myxoid components (Antoni B areas) [9, 12]. Only immunohistochemical analysis of the surgical specimen can differentiate these types of mesenchymal tumors [4, 10], with GISTs staining for CD34 and CD117, c-kit [4], and schwannomas for the S-100 protein [4, 6, 8], vimentin and glial fibrillary acidic protein [13]. Some molecular changes have been identified in this type of tumor such as lack of c-kit (CD117), PDGFRA mutations [3, 8], chromosome 22 monosomy, and polyploidy of chromosomes 2 and 18 [10].
Post resection, these patients have an excellent prognosis, with rare recurrence [4, 5, 8, 13]. At the moment, there is no current recommendation for follow-up with a CT scan, given the benefit and risk of exposure to ionizing radiation [15]. However, clinical follow-up is important to detect any symptomatic recurrence.
This case report illustrates a rare etiology of gastrointestinal bleeding, specifically a gastric schwannoma, which is a rare type of benign gastric tumor. When observing a patient for this type of symptom, gastric schwannoma may not be immediately considered. The thorough diagnostic evaluations and the comprehensive approach reported are crucial for ensuring appropriate treatment planning, thereby improving patient outcomes. The complete excision of the schwannoma in this case, facilitated by minimally invasive laparoscopic surgery, further demonstrates the advancements in surgical techniques that allow for effective management of such rare cases with minimal patient morbidity.
In conclusion, gastric schwannomas should be considered as a potential cause of digestive bleeding. The benign nature of these tumors generally leads to a favorable outcome, particularly when they are accurately diagnosed and completely resected.
Learning points
Gastric schwannoma is a rare cause of upper gastrointestinal bleeding, sometimes forgotten in the differential diagnosis of the causes of this symptom. However, it should be considered, as its treatment is generally easy to perform and has an excellent prognosis after resection.
Acknowledgments
The authors wish to thank the patient for his collaboration in carrying out this case report.
Financial Disclosure
The authors have no funding sources to report.
Conflict of Interest
The authors have no conflict of interest to declare.
Informed Consent
Informed consent was obtained from the patient.
Author Contributions
The authors confirm their contribution to the paper as follows: Daniela Pato Pais: corresponding and first author, study conception and design, data collection, article writing and literature revision of the theme in question. Margarida Luis, Ines Bertao Colaco, Sara Andrade: data analysis and interpretation of results and final approval of the manuscript. Nuno Azenha, Ana Couceiro, Jose Valente Cecilio: critical revision of the manuscript for important intellectual content, final approval of the version to be published.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Raber MH, Ziedses des Plantes CM, Vink R, Klaase JM. Gastric schwannoma presenting as an incidentaloma on CT-Scan and MRI. Gastroenterology Res. 2010;3(6):276-280.
doi pubmed - Zhong Z, Xu Y, Liu J, Zhang C, Xiao Z, Xia Y, Wang Y, et al. Clinicopathological study of gastric schwannoma and review of related literature. BMC Surg. 2022;22(1):159.
doi pubmed - Tao K, Chang W, Zhao E, Deng R, Gao J, Cai K, Wang G, et al. Clinicopathologic Features of Gastric Schwannoma: 8-Year Experience at a Single Institution in China. Medicine (Baltimore). 2015;94(45):e1970.
doi pubmed - Yoon W, Paulson K, Mazzara P, Nagori S, Barawi M, Berri R. Gastric schwannoma: a rare but important differential diagnosis of a gastric submucosal mass. Case Rep Surg. 2012;2012:280982.
doi pubmed - Wu X, Li B, Zheng C, He X. Clinical characteristics and surgical management of gastrointestinal schwannomas. Biomed Res Int. 2020;2020:9606807.
doi pubmed - Daimaru Y, Kido H, Hashimoto H, Enjoji M. Benign schwannoma of the gastrointestinal tract: a clinicopathologic and immunohistochemical study. Hum Pathol. 1988;19(3):257-264.
doi pubmed - Bruneton JN, Drouillard J, Roux P, Ettore F, Lecomte P. Neurogenic tumors of the stomach. Report of 18 cases and review of the literature. Rofo. 1983;139(2):192-198.
doi pubmed - Voltaggio L, Murray R, Lasota J, Miettinen M. Gastric schwannoma: a clinicopathologic study of 51 cases and critical review of the literature. Hum Pathol. 2012;43(5):650-659.
doi pubmed - Hughes MJ, Thomas JM, Fisher C, Moskovic EC. Imaging features of retroperitoneal and pelvic schwannomas. Clin Radiol. 2005;60(8):886-893.
doi pubmed - Pu C, Zhang K. Gastric schwannoma: a case report and literature review. J Int Med Res. 2020;48(9):300060520957828.
doi pubmed - Sreevathsa MR, Pipara G. Gastric schwannoma: a case report and review of literature. Indian J Surg Oncol. 2015;6(2):123-126.
doi pubmed - Lauricella S, Valeri S, Masciana G, Gallo IF, Mazzotta E, Pagnoni C, Costanza S, et al. What about gastric schwannoma? A review article. J Gastrointest Cancer. 2021;52(1):57-67.
doi pubmed - Shah AS, Rathi PM, Somani VS, Mulani AM. Gastric schwannoma: a benign tumor often misdiagnosed as gastrointestinal stromal tumor. Clin Pract. 2015;5(3):775.
doi pubmed - He MY, Zhang R, Peng Z, Li Y, Xu L, Jiang M, Li ZP, et al. Differentiation between gastrointestinal schwannomas and gastrointestinal stromal tumors by computed tomography. Oncol Lett. 2017;13(5):3746-3752.
doi pubmed - Hong X, Wu W, Wang M, Liao Q, Zhao Y. Benign gastric schwannoma: how long should we follow up to monitor the recurrence? A case report and comprehensive review of literature of 137 cases. Int Surg. 2015;100(4):744-747.
doi pubmed - Koh YX, Chok AY, Zheng HL, Tan CS, Chow PK, Wong WK, Goh BK. A systematic review and meta-analysis comparing laparoscopic versus open gastric resections for gastrointestinal stromal tumors of the stomach. Ann Surg Oncol. 2013;20(11):3549-3560.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.