| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com |
Case Report
Volume 16, Number 1, January 2025, pages 23-27
Pulmonary Mucormycosis in an Older Acute Myeloid Leukemia Patient
Bing Qing Luoa, Xiao Yan Tana, Ying Chena, Lin Chena, b
aDepartment of Hematology, The Second Affiliated Hospital, Chongqing Medical University, Jiangnan, Chongqing, China
bCorresponding Author: Lin Chen, Department of Hematology, The Second Affiliated Hospital, Chongqing Medical University, Jiangnan, Chongqing, China
Manuscript submitted October 3, 2024, accepted November 6, 2024, published online November 23, 2024
Short title: Pulmonary Mucormycosis in an Old AML Patient
doi: https://doi.org/10.14740/jmc5042
| Abstract | ▴Top |
Mucormycosis is a rare but fatal opportunistic fungal infection. Patients with hematologic malignancies who use immunosuppressant and glucocorticoid extensively are susceptible to mucormycosis. We report a case of an older patient with acute myeloid leukemia (AML) who was infected with pulmonary mucormycosis during chemotherapy. With a good balance of chemotherapy-induced myelosuppression and control of Mucorales infection, the patient got a complete remission of leukemia by the combination of azacitidine and venetoclax, and pulmonary mucormycosis was well controlled. But due to poor compliance, the patient died of asphyxiation as a result of aspiration of pulmonary necrosis-like substances after discharge. To our knowledge, this is the first case report of an older patient with AML complicated with pulmonary mucormycosis dying of asphyxia from pulmonary necrosis-like substances. Our article seeks to raise awareness of proper precautions to be taken in the management mucormycosis.
Keywords: Pulmonary mucormycosis; Older; Acute myeloid leukemia; Venetoclax
| Introduction | ▴Top |
Mucormycosis has been notorious for its aggressive angioinvasion and high mortality. Rarity, nonspecific clinical manifestations and negative blood culture make mucormycosis often misdiagnosed. Pulmonary mucormycosis is the second most common type of mucormycosis, with a mortality rate of up to 80% [1]. Patients with hematologic malignancies under chemotherapy always suffering immunosuppression are susceptible to pulmonary mucormycosis. Compared with young acute myeloid leukemia (AML) patients, older AML patients have more underlying diseases, poor performance state, decreased function of multiple organs, more complex karyotypes and gene mutations with poor prognosis, making allogeneic stem cell transplantation and high-dose chemotherapy unsuitable for part of older AML patients. Survival in older AML patients remains poor.
Here we report an older AML patient who was infected with Mucorales during induction chemotherapy.
| Case Report | ▴Top |
A 68-year-old male initially presented with neutropenia, dyspnea and tiredness. He had a history of pulmonary tuberculosis and had taken rifampicin and ethambutol for 6 months. Blood routine test demonstrated a white blood cell (WBC) count of 7.81 × 109/L with an absolute neutrophil count (ANC) of 0.09 × 109/L, hemoglobin of 68 g/L, and platelet (PLT) count of 77 × 109/L. Initial bone marrow smear revealed a hypercellular marrow with 92% blasts, and flow cytometry confirmed that they were myeloid (CD34+, CD117+, CD33+, MPO+, CD13+, CD38+), raising concern for AML-M1. Cytogenetic studies were remarkable for normal karyotype with DNMT3A and IDH2 mutations. He got an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 3. Shortly after admission, the patient developed fever, and sputum culture suggested Mycoplasma pneumoniae infection. Chest computed tomography (CT) showed progressed areas of consolidation in the middle-lower lobe of the right lung (Fig. 1a). Levofloxacin was selected according to the results of drug susceptibility testing. Considering the severity of infection and high blasts count, the patient was given a 7-day regimen of azacytidine (AZA) 100 mg/day as the “bridging chemotherapy”. The patient was discharged after the pulmonary infection was controlled.
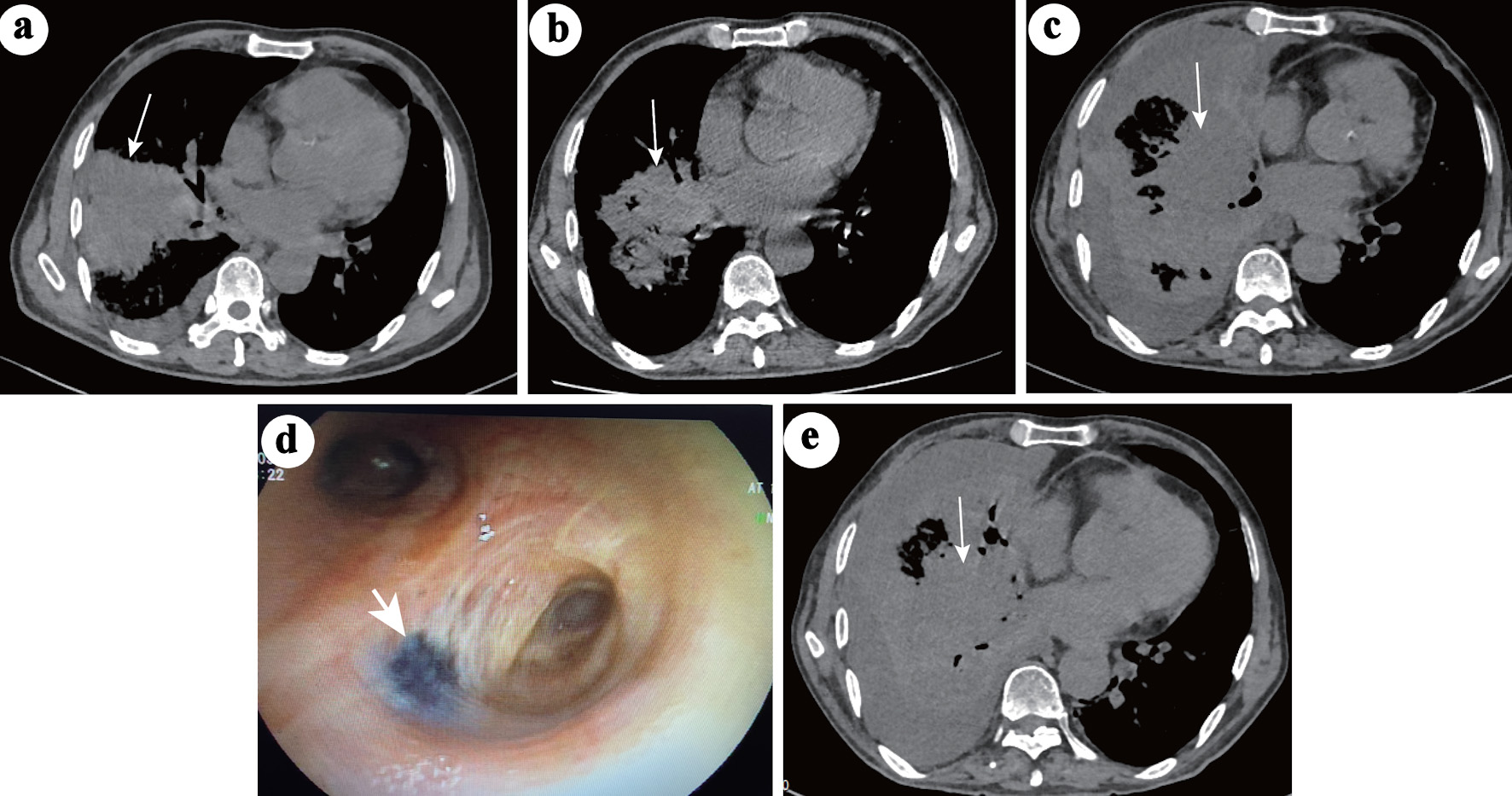 Click for large image | Figure 1. Chest CT changes during the course of pneumonia. (a) Large areas of consolidation (white arrow) in the middle-lower right lobe and pleural effusion during the first hospitalization when the patient was infected with Mycoplasma pneumoniae. (b) On the second admission, the consolidation (white arrow) in the right middle-lower lobe was significantly absorbed and decreased, and the pleural effusion disappeared. (c) After 2 weeks of nonspecific antifungal treatment, the consolidation (white arrow) in the right lobe progressed, accompanied by obstructive inflammation and lymphangitis. Pleural effusion also appeared. (d) Video bronchoscopy image showing a black fungating mass in the right principal bronchus (white arrow). (e) No further progression in the right lobe (white arrow) after 26 days of anti-mucor therapy. CT: computed tomography. |
One month later, the patient was admitted for the next cycle of chemotherapy. Blood routine test demonstrated WBC of 20.58 × 109/L, ANC of 0.69 × 109/L, hemoglobin of 28 g/L and PLT of 99 × 109/L. Reexamination of bone marrow smears showed 72% blasts, and chest CT still indicated pneumonia but less than before (Fig. 1b). The patient got a fever of 38.5 °C on day 2 of admission (d-2), and levofloxacin and tigecycline were used. On day 4 of admission (d1), the patient was started on a 7-day regimen of AZA (100 mg/day) and 28 days of venetoclax. Neutropenia (nadir WBC = 0.19 × 109/L and ANC 0.04 × 109/L) and thrombocytopenia (nadir PLT count = 12 × 109/L) were developed 4 days after the start of chemotherapy (d4). The patient developed gradual exaggeration of breathlessness, cough and fever (with a maximum body temperature of 39.2 °C). Repeated blood cultures, throat swabs, serum galactomannan antigen (GM) test and 1,3-b-D glucan test (G test) were negative. Due to the low PLT count, bronchoalveolar lavage (BAL) was not performed. Biapenem, ceftizoxime, piperacillin-tazobactam, meropenem and colistimethate sodium, were given either singly or in combination, successively, as empirical antibacterial drugs; caspofungin, posaconazole (Pcz), and voriconazole were successively supplemented as antifungal drugs, but the PS of the patient did not improve significantly. On auscultation, breathing sounds of the right lobe gradually become undetectable. Chest CT indicated the progression of pneumonia (Fig. 1c). The dose of venetoclax began to reduce at d11 (d1: 100 mg, d2: 200 mg, d3 - d10: 400 mg, d11 - d15: 200 mg, d15 - d28: 100 mg) due to severe neutropenia, thrombocytopenia and use of inhibitor of CYP3A (Pcz, voriconazole), but the 28-day course was never interrupted. On d18, the patient’s blood count began to recover, and PLT count reached 199 × 109/L, so that BAL was performed immediately. Video-bronchoscopy showed that an endobronchial fungating mass leading to luminal obstruction in the right principal bronchus (Fig. 1d). Both the fungal culture and sequencing from BAL fluid were positive for Rhizopus microsporus. Amphotericin B cholesteryl sulfate complex for injection (ABCD) 3 mg/kg once a day (qd) was initiated on d21. Pcz 400 mg twice a day (bid) and meropenem 1 g every 8 h (q8h) was continued. Persistent fever subsided within 24 h after using ABCD. To improve the antifungal effect [2], ABCD nebulizer therapy was added on d24. On d36, the antibiotic was downgraded from meropenem to cefazoxime for bacterial prophylaxis. Cough was the most prominent symptom in this patient. Methylprednisolone was effective in relieving the cough and was not completely discontinued until the d50. Upon hematologic recovery, clearance of peripheral blasts was noted on d25. Twenty-six days later, the patient’s clinical symptoms and performance state were significantly improved, and chest CT showed no further progression (Fig. 1e). Blasts counts were reduced to 2% in bone marrow smears on d54. Antifungal therapy (ABCD + Pcz) was continued for a total of 39 days. The clinical course and timeline of the treatment course were summarized in Figure 2. According to the plan, the patient needed to undergo the next cycle of chemotherapy in a week, but for personal reasons, the patient asked to go home for a rest. The patient was discharged with stepdown treatment of Pcz 400 mg orally bid. Unfortunately, the patient died 7 days after discharge. The family said that the patient was generally fine at home, but suddenly developed a violent cough and coughed up large amounts of dark red “necrosis-like substances” and died at home within minutes. No autopsy was performed, but the patient is presumed to have died of asphyxiation due to aspiration of pulmonary necrosis-like substances.
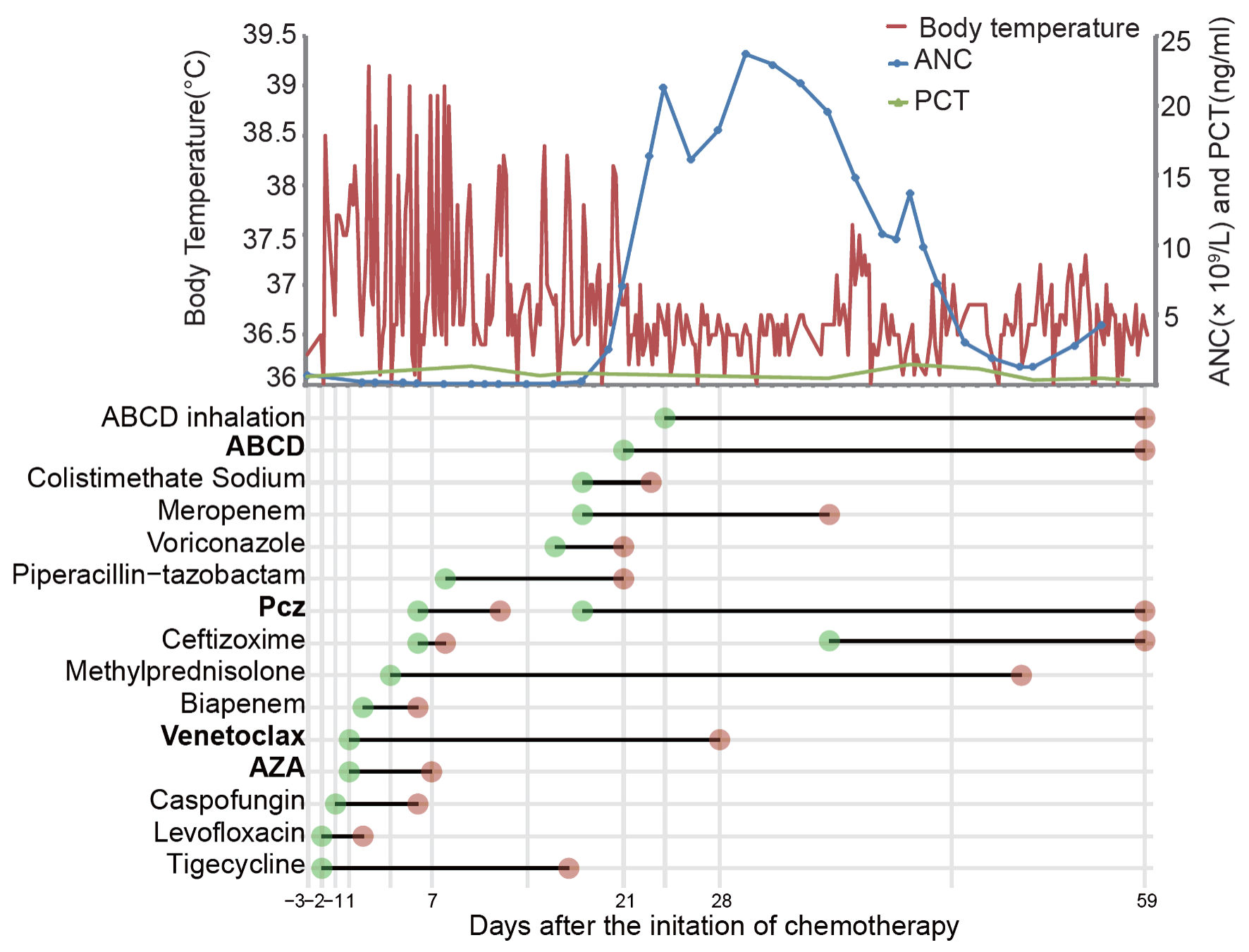 Click for large image | Figure 2. The clinical and treatment course of the patient. The first day of chemotherapy is considered as day 1 (d1). ANC: absolute neutrophil count; PCT: procalcitonin; AZA: azacytidine; Pcz: posaconazole; ABCD: amphotericin B cholesteryl sulfate complex for injection. |
| Discussion | ▴Top |
Mucorales is one of the most important opportunistic fungal infections after candidiasis and aspergillosis, and it often infects patients with severe underlying diseases. In acute leukemia, voriconazole is increasingly used to prevent and treat aspergillosis, which also selectively increases the risk of mucormycosis [3, 4]. Mucorales organisms are angio-invasive, causing intravascular thrombosis and infarction of surrounding tissues. After being infected with Mucorales, the body’s immune cells eliminate it by producing oxidative metabolites and defensins. Neutrophil dysfunction or neutropenia in patients with leukemia cannot produce sufficient immune mediators against Mucorales. Mucorales mostly invades naso-orbito-brain, followed by lung and skin [5, 6]. Patients with hematologic malignancies most often suffer from pulmonary type [7].
Clinical presentations of pulmonary mucormycosis are not specific, such as persistent hyperpyrexia, cough, dyspnea, hemoptysis, etc. Imaging findings include local consolidation, opacity, nodule, cavitation, reverse halo sign, pleural effusion, etc. Multiple pulmonary nodules (> 10) have some significance for pulmonary mucormycosis but are also nonspecific [8]. The patient had been infected with tuberculosis and Mycoplasma pneumoniae prior to mucormycosis infection. Pulmonary tuberculosis manifested as multiple pulmonary nodules, while Mycoplasma pneumoniae manifested as consolidation in the right lobe, which disturbed the determination of pulmonary mucormycosis. Among the pathogenic Mucorales, the genera Rhizopus is the most common. Metagenomics next generation sequencing (mNGS) from BAL fluid has unique advantages in the detection of rare pathogens due to its high sensitivity and lower sample contamination [9]. It shows at least 81% sensitivity of detecting Mucorales DNA [10]. However, bronchoscopy is still an invasive method that requires a certain number of PLT count and normal coagulation function. The indications for bronchoscopy should be relaxed appropriately to facilitate earlier diagnosis.
The mortality of pulmonary mucormycosis in China is close to 40% [11]. Bacteremia is the only clinical feature associated with mortality, while 20% of pulmonary mucormycosis can be complicated with bacteremia [8]. In this case, the patient experienced myelosuppression after chemotherapy, but no bacterial infection occurred during the course of the disease through a combination of antifungal and prophylactic antibacterial treatment. Surgical intervention is instrumental for mucormycosis, however, severe thrombocytopenia and particular sites of infection like the lungs and throat make surgical resection challenging. Antifungal therapy includes amphotericin, esaconazole and Pcz as monotherapy or in combination. Although there is no clear evidence that combination is superior to single agents, amphotericin combined with Pcz is commonly used in clinical practice. The duration of antifungal therapy for mucormycosis is unknown, but typically ranges from months to years [1]. Venetoclax combined with hypomethylating agents (HMAs) (decitabine, AZA) for older AML patients who are unfit for intense chemotherapy has a complete remission (CR) rate of up to 71% [12, 13]. In this case, the first course of AZA monotherapy served as a “bridging chemotherapy”, allowing for the addition of venetoclax in the next course when the pneumonia was controlled. Unfortunately, the patient developed pneumonia again shortly after the initiation of second chemotherapy. The pneumonia progressed rapidly during the myelosuppression phase. Chest CT indicated obstructive pneumonia in the right lobe. The chemotherapy was not interrupted in spite of pancytopenia and severe infection. Balancing myelosuppression caused by chemotherapy and controlling Mucorales infection is necessary, but rapid remission of AML and restoration of normal neutrophils through chemotherapy are key to stop the vicious cycle [14]. In this case, intensive supportive care and a modest reduction in venetoclax dose depending on the patient’s condition resulted in CR of AML, and pulmonary mucormycosis was also controlled.
The patient died of accidental asphyxiation at home due to inhalation of necrosis-like substances in the lungs, which was rarely reported in AML patients with mucormycosis. In terms of complications of lung mucormycosis, most studies have focused on hemoptysis, but little attention has been paid to asphyxia caused by the excretion of necrotic materials from the lungs. The poor PS and severe thrombocytopenia rendered the patient inoperable when mucormycosis was initially diagnosed. As his condition improved, surgical debridement was not planned positively. It is a painful lesson that lung debridement should be considered even when the lesion is effectively controlled. More importantly, during the rehabilitation of mucormycosis, the risk of suffocation of necrotic materials needs to be closely monitored, especially when debridement has not been performed.
Learning points
Patients with hematologic malignancy, especially during chemotherapy, are susceptible to mucormycosis. When multiple antibiotics, anti-candida and anti-aspergillus therapies are ineffective, mucormycosis should be highly suspected. For older AML patients diagnosed with mucormycosis during chemotherapy, adherence to the chemotherapy and restoration of neutrophils is key to breaking the vicious cycle. Even if mucormycosis is under control, debridement should be considered, and suffocation caused by necrotic materials should be well managed and prevented.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors have no conflict of interest to report.
Informed Consent
Written informed consent was obtained from the patient’s family for publication of this case report.
Author Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Xiao Yan Tan. The first draft of the manuscript was written by Bing Qing Luo. Ying Chen and Lin Chen performed the final review, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
| References | ▴Top |
- Steinbrink JM, Miceli MH. Mucormycosis. Infect Dis Clin North Am. 2021;35(2):435-452.
doi pubmed - Kothari S, Kefalos SG, Hages ND, Corcoran TE, Husain S. Preclinical studies of the nebulized delivery of Liposomal Amphotericin B. J Aerosol Med Pulm Drug Deliv. 2022;35(6):307-312.
doi pubmed - Colovic N, Arsic-Arsenijevic V, Barac A, Suvajdzic N, Lekovic D, Tomin D. Mucormycosis of the paranasal sinuses in a patient with acute myeloid leukemia. Srp Arh Celok Lek. 2016;144(11-12):657-660.
pubmed - Imhof A, Balajee SA, Fredricks DN, Englund JA, Marr KA. Breakthrough fungal infections in stem cell transplant recipients receiving voriconazole. Clin Infect Dis. 2004;39(5):743-746.
doi pubmed - Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634-653.
doi pubmed - A SS, Pishdad G, Bolandparvaz S. Epidemiology and clinical characteristics of mucormycosis in patients with leukemia; a 21-year experience from Southern Iran. Bull Emerg Trauma. 2014;2(1):38-43.
pubmed - Pak J, Tucci VT, Vincent AL, Sandin RL, Greene JN. Mucormycosis in immunochallenged patients. J Emerg Trauma Shock. 2008;1(2):106-113.
doi pubmed - Lin E, Moua T, Limper AH. Pulmonary mucormycosis: clinical features and outcomes. Infection. 2017;45(4):443-448.
doi pubmed - Kumar S, Joshi D. Pulmonary mucormycosis in immunocompetent hosts diagnosed by bronchioalveolar lavage. BMJ Case Rep. 2021;14(4).
doi pubmed - Millon L, Herbrecht R, Grenouillet F, Morio F, Alanio A, Letscher-Bru V, Cassaing S, et al. Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: retrospective analysis of 44 cases collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF). Clin Microbiol Infect. 2016;22(9):810.e1-e8
doi pubmed - Spatafora JW, Chang Y, Benny GL, Lazarus K, Smith ME, Berbee ML, Bonito G, et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia. 2016;108(5):1028-1046.
doi pubmed - DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, Frankfurt O, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7-17.
doi pubmed - DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, Konopleva M, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med. 2020;383(7):617-629.
doi pubmed - Aftandilian C, Eguiguren L, Mathew R, Messner A. Mucormycosis diagnosed during induction chemotherapy in five pediatric patients with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2019;66(9):e27834.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.