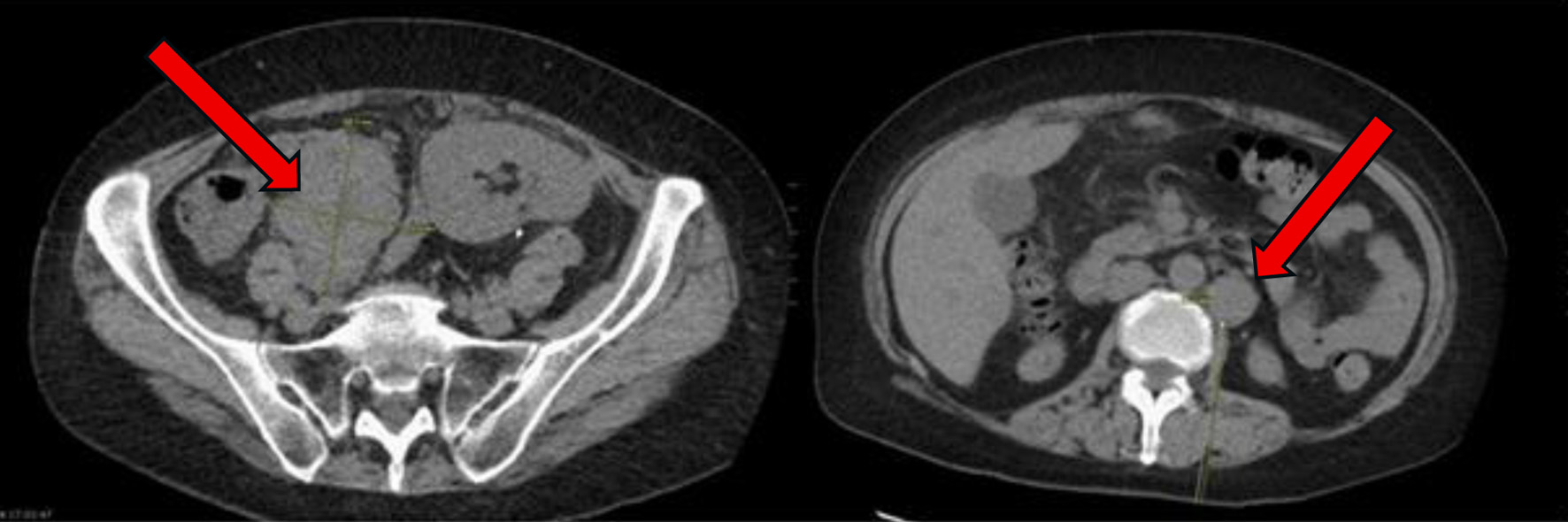
Figure 1. At time of initial presentation, CT abdomen and pelvis showing increase in size of the abdominal and pelvic lymph nodes (arrows) suspicious for post-transplant lymphoproliferative disorder. CT: computed tomography.
| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com |
Case Report
Volume 17, Number 1, January 2026, pages 1-8
Refractory Epstein-Barr Virus-Negative Diffuse Large B-Cell Lymphoma Post-Transplant Lymphoproliferative Disorder in a Renal Transplant Patient Treated With Bispecific Antibody Therapy
Figures

Table
| Timeline | Clinical events and findings | Management | Outcome |
|---|---|---|---|
| “M” represents months in the timeline. “W” represents weeks in the timeline. The table begins with the patient’s kidney transplant and early symptoms then arriving “day 0” with our inpatient team’s first encounter. The following rows detail her treatment course and disease progression. CAR: chimeric antigen receptor; CMV: cytomegalovirus; CRS: cytokine release syndrome; CT: computed tomography; DLBCL: diffuse large B-cell lymphoma; EBV: Epstein-Barr virus; EGD: esophagogastroduodenoscopy; ESRD: end-stage renal disease; GI: gastrointestinal; ICU: intensive care unit; PET: positron emission tomography; PTLD: post-transplant lymphoproliferative disorder; R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; R-GemOx: rituximab, gemcitabine, oxaliplatin. | |||
| 30 M prior to initial presentation | Deceased donor kidney transplant for ESRD (hypertensive nephrosclerosis). | Immunosuppression with tacrolimus, mycophenolate, prednisone. | Stable graft function post-transplant. |
| 6 M prior to initial presentation | Progressive abdominal discomfort, early satiety, unintentional weight loss. | Symptom monitoring. | Worsening symptoms over months. |
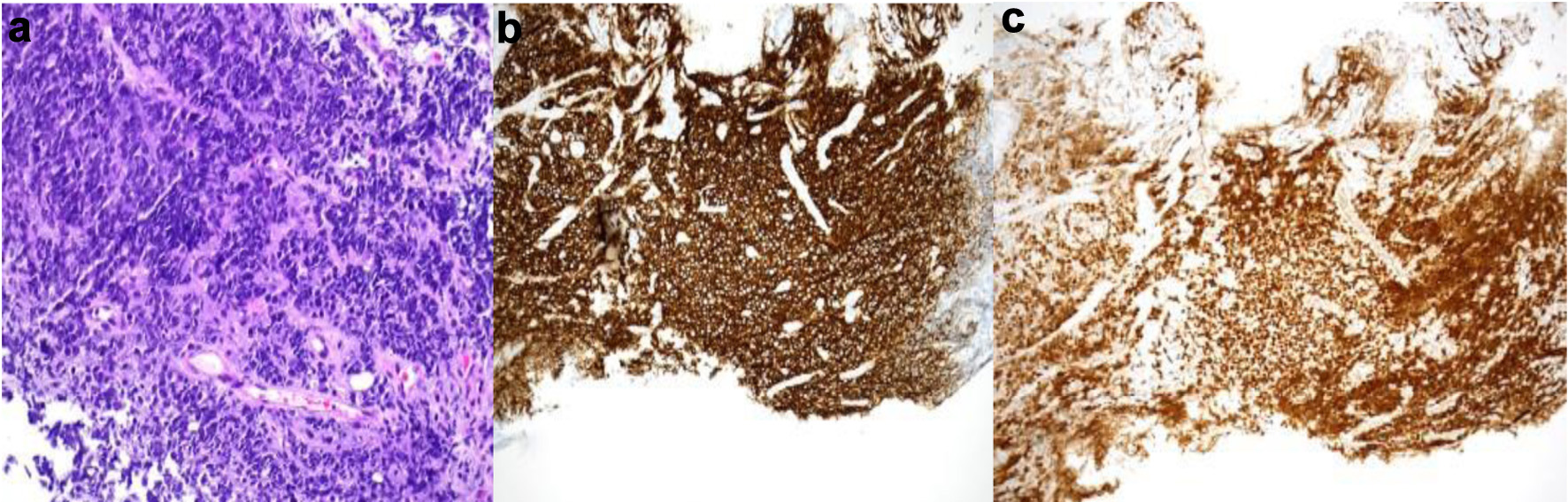
| Initial presentation, “day 0” | Abdominal pain and fatigue. CT abdomen and pelvis showing increase in size of the abdominal and pelvic lymph nodes suspicious for post-transplant lymphoproliferative disorder (Fig. 2). | Retroperitoneal lymph node biopsy. | Diagnosed with EBV-negative DLBCL, germinal center B-cell subtype PTLD. |
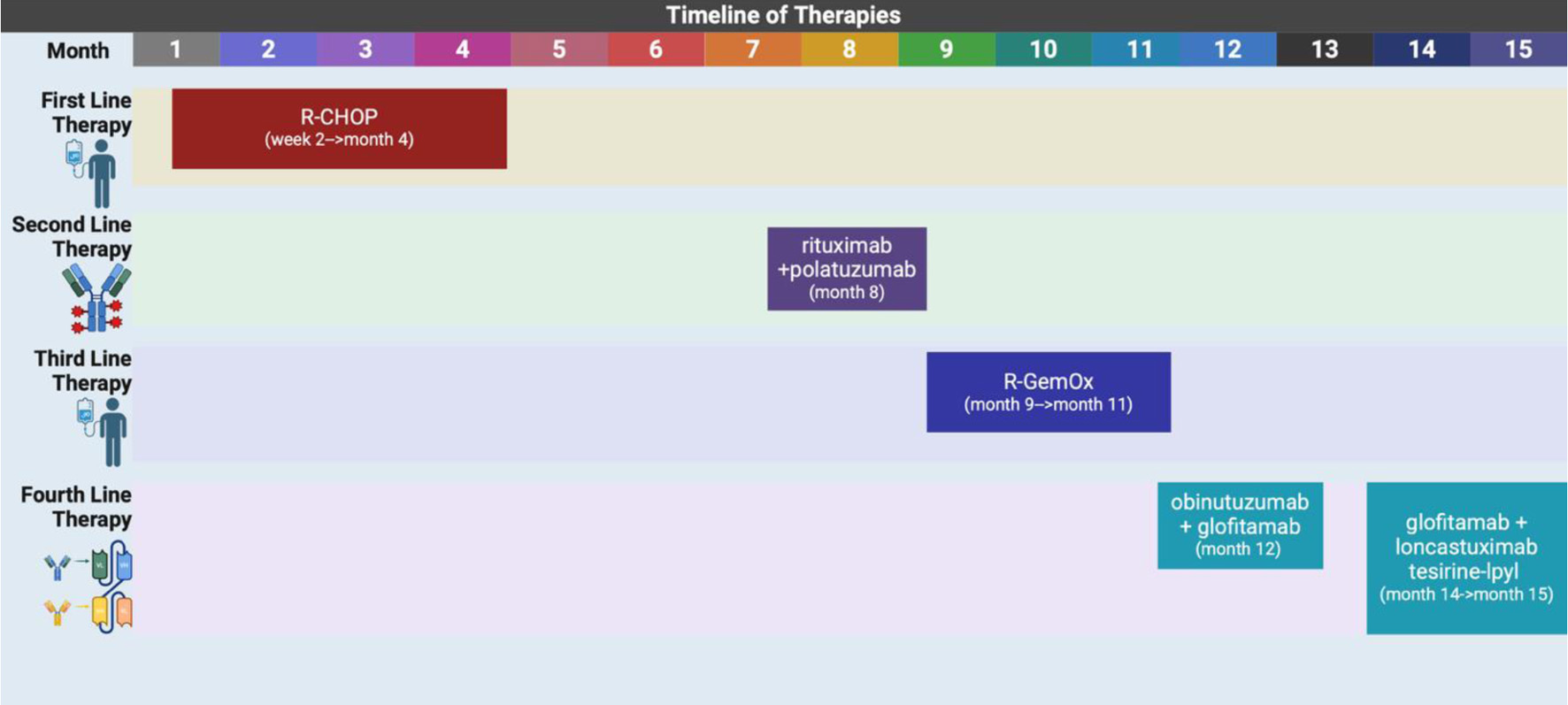
| 2 W after presentation | Initiated first cycle of R-CHOP. | Rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone. | Mild neutropenia, partial radiographic response after two cycles. |
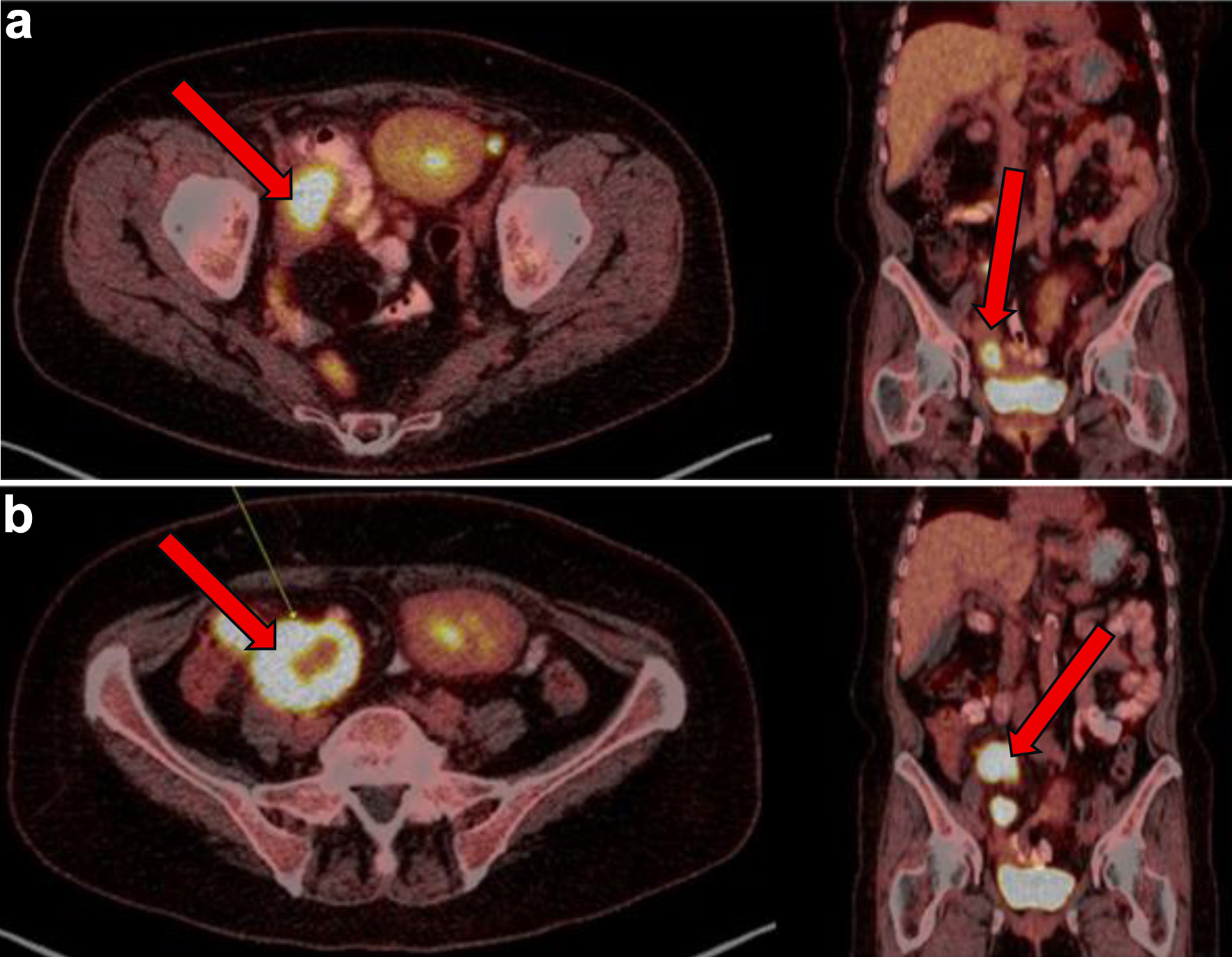
| 4 M after presentation | Disease progression post six cycles of R-CHOP. PET: new pelvic nodes, bowel lesions (Fig. 3). | Biopsy of right pelvic node: residual DLBCL with necrosis, high Ki-67, GC phenotype. | Confirmed refractory disease. |
| 8 M after presentation | Started on rituximab + polatuzumab vedotin (delayed due to hospitalizations). | Chemotherapy initiated. | Admitted to ICU with septic shock post-cycle. |
| 8 - 9 M after presentation | EGD/colonoscopy: gastric ulcer (DLBCL), CMV colitis. | Multidisciplinary discussion; suppressive antibiotics. | Decision to continue therapy. |
| 9 - 11 M after presentation | Four cycles of R-GemOx. | Tolerated treatment initially. | CT in October: disease progression with worsening abdominal pain. |
| 12 M after presentation | Bridging therapy initiated: obinutuzumab + glofitamab for CAR T-cell consideration. | Admitted for CRS monitoring after first dose. | Discharged, tolerated initial treatment. |
| 13 - 14 M after presentation | Polatuzumab added. Recurrent admissions for GI toxicity (C. difficile colitis, pain). | Supportive care, pain control. | CT: tumor progression (up to 11.9 cm mass), new ascites. |
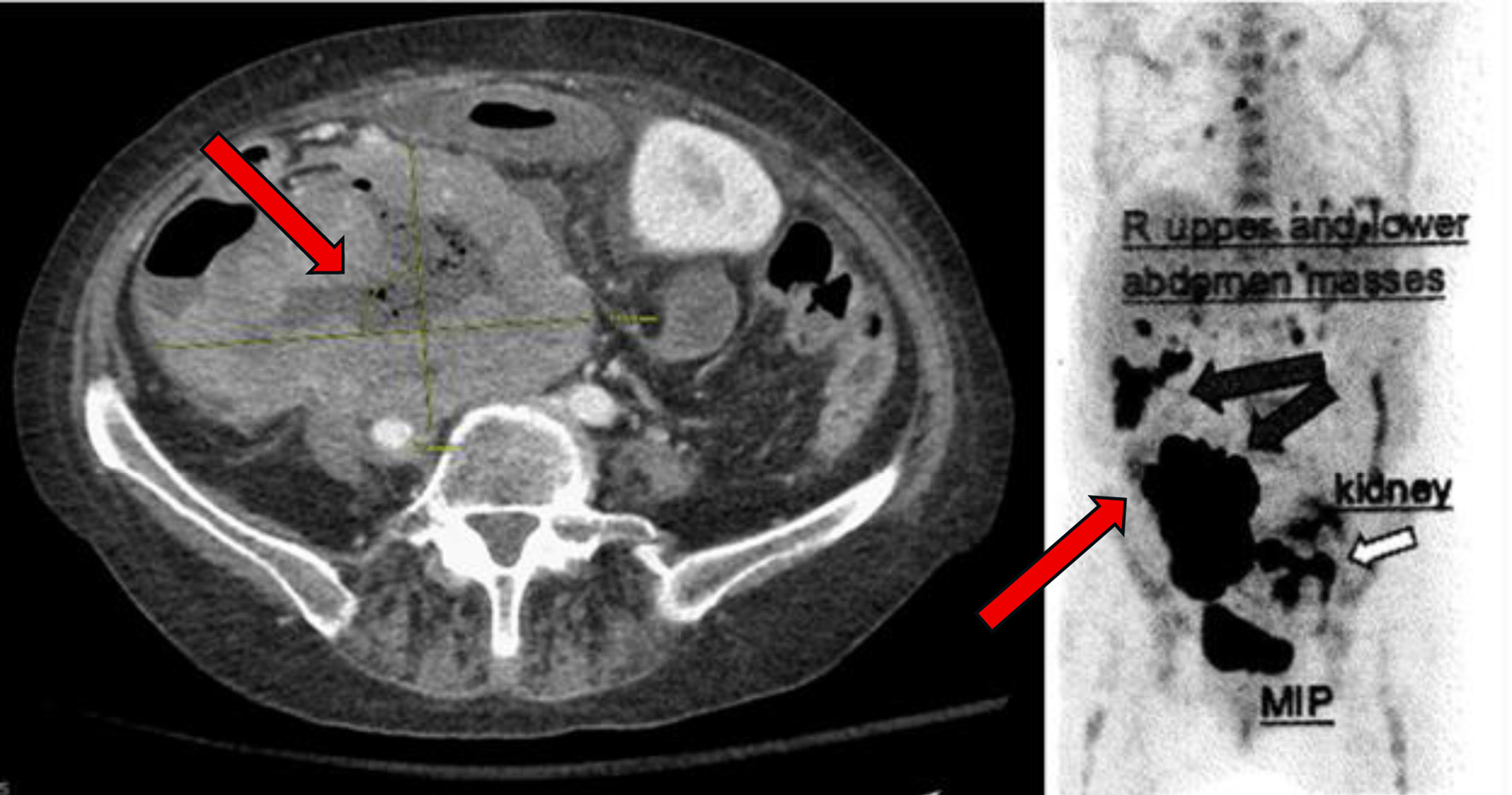
| 14 - 15 M after presentation | PET scan confirms disease progression (Fig. 4). | Started glofitamab + loncastuximab tesirine-lpyl (LOTIS-7 trial). | Admitted soon after first dose with pain, fevers. |
| 15 M after presentation | Emotional distress, ongoing symptoms. Imaging: no new disease. | Goals-of-care discussion. Transitioned to hospice care. | Patient chose to stop treatment. |