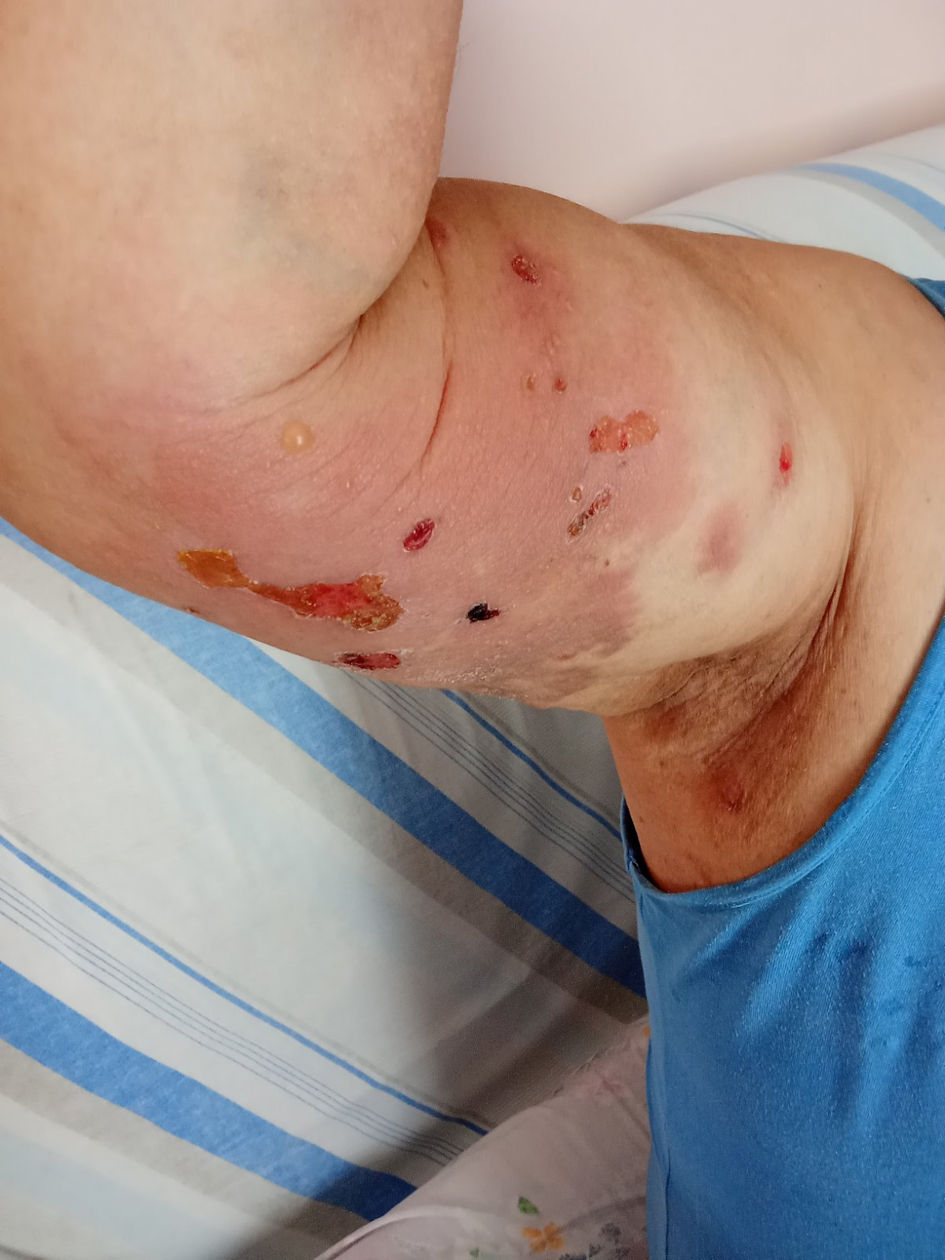
Figure 1. Tense bulla with clear fluid on the right upper extremity of the patient. Note the erythematous rash with erosions and blistering.
| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com |
Case Report
Volume 16, Number 2, February 2025, pages 69-76
An Adverse Double-Hit by Pembrolizumab: A Case Report of Bullous Pemphigoid and Pneumonitis
Figures

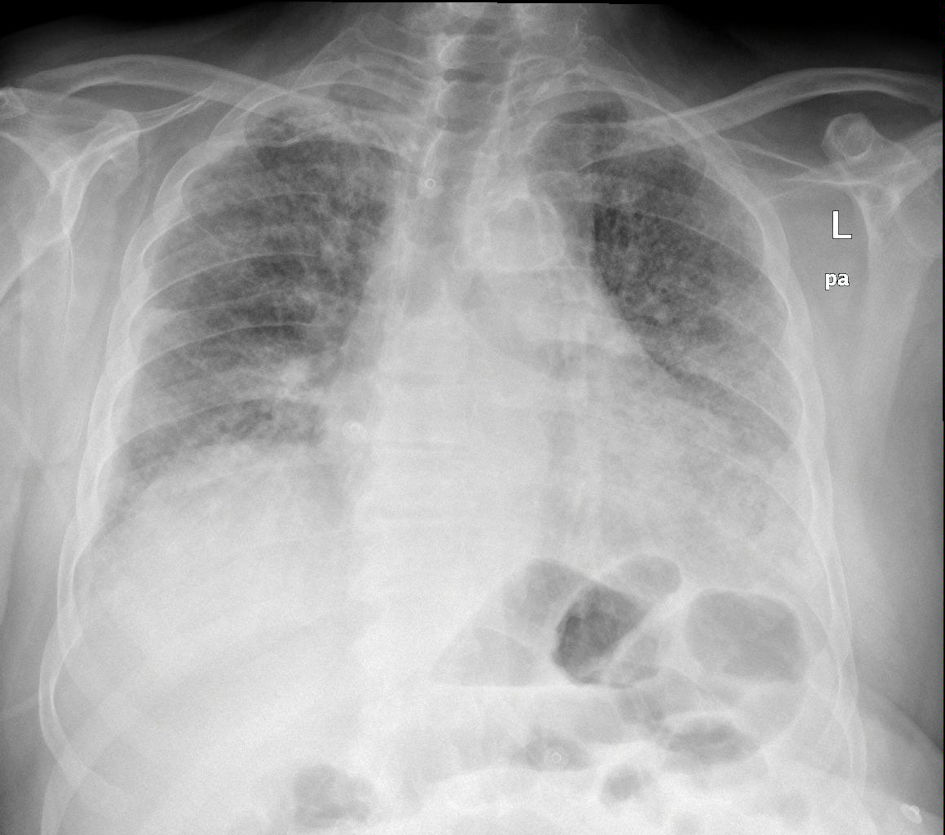
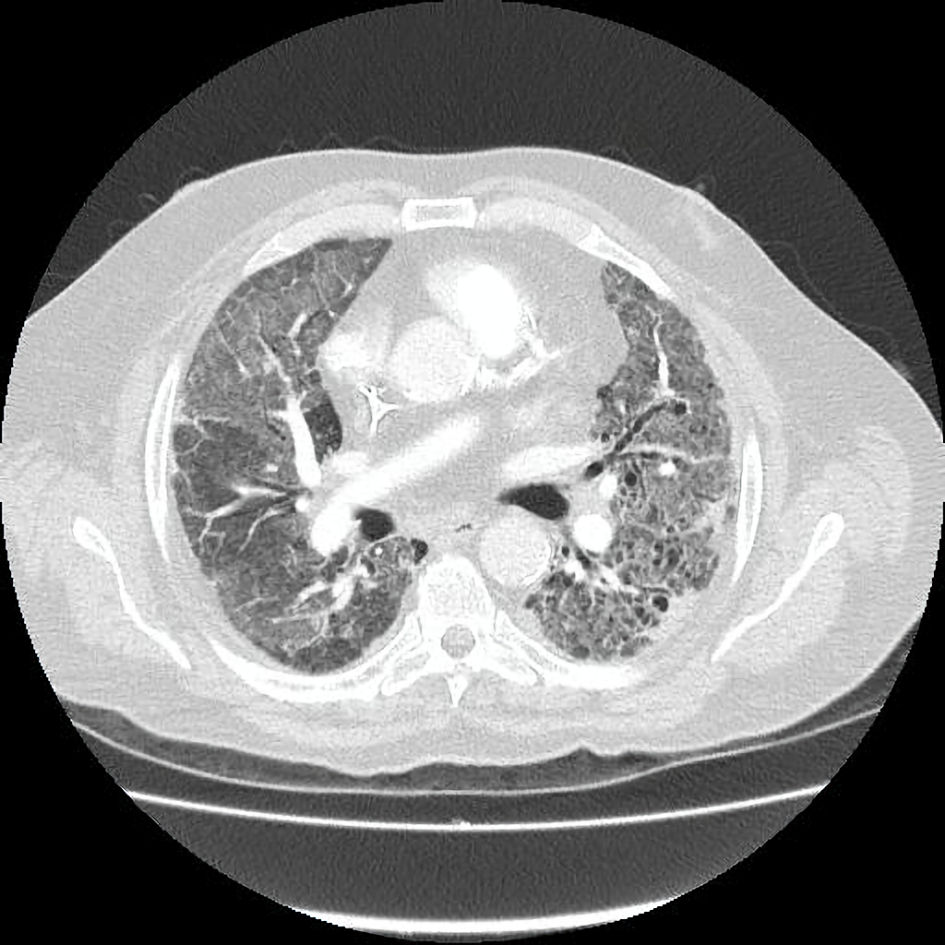
Tables
| Laboratory parameters | On admission | Reference range |
|---|---|---|
| ED: emergency department. | ||
| Blood pressure | 133/74 mm Hg | |
| Heart rate | 107 | |
| Respiratory rate | 30 | |
| Temperature | 37.6 °C | |
| Oxygen saturation | 75% | |
| Electrocardiogram | Normal | |
| High-sensitivity troponin I | 150 pg/mL | 0 - 34 pg/ml |
| D-dimers | > 20 mg/L | 0 - 0.5 mg/L |
| Lactate dehydrogenase | 400 U/L | 120 - 230 U/L |
| C-reactive protein | 43.6 mg/dL | < 0.5 mg/dL |
| White blood cells (/µL) | 17,960 | 4,000 - 11,000 |
| Neutrophils | 86% | 50-70% |
| Lymphocytes | 6% | 20-40% |
| Hemoglobin | 8.6 g/dL | 13 - 17 g/dL |
| Platelets (/µL) | 449,000 | 150,000 - 400,000 |
| Severity of BP secondary to immunotherapy | Discontinuation of immunotherapy | Treatment |
|---|---|---|
| N/A: not available. | ||
| Grade 1: < 10% of body surface area (BSA) | No | Consider oral corticosteroids (0.5 - 1 mg/kg/day) and non-steroidal immunosuppressants |
| Grade 2: 10-30% of BSA | No | Treatment as above |
| Grade 3: > 30% of BSA | Yes | Add oral or intravenous corticosteroids (1 - 2 mg/kg/day) |
| Consider non-steroidal immunosuppressants | ||
| Grade 4: > 30% of BSA and life-threatening complications, e.g., fluid/electrolyte disorders, intensive care is necessary | Yes | Add intravenous corticosteroids (1 - 2 mg/kg/day) |
| Consider non-steroidal immunosuppressants | ||
| All grades | N/A | Topical corticosteroids, topical emollients, oral antihistamines |
| Tapering of systemic corticosteroids for at least 1 month | ||
| Non-steroidal immunosuppressants: dapsone, doxycycline, mycophenolate mofetil, cyclophosphamide, azathioprine, omalizumab, dupilumab, rituximab, intravenous immunoglobulin | ||
| Severity of pneumonitis secondary to immunotherapy | Definition | Discontinuation of immunotherapy | Administration of corticosteroids |
|---|---|---|---|
| P. jirovecii: Pneumocystis jirovecii; N/A: not available. | |||
| Grade 1 | Subclinical; observation without treatment | Temporary | No |
| Grade 2 | Mild presentation; outpatient treatment indicated | Temporary | Prednisone 1 - 2 mg/kg/day with a taper; consider gastrointestinal and P. jirovecii prophylaxis |
| Grade 3 | Severe presentation; inpatient treatment indicated | Permanent | Prednisone 1 - 2 mg/kg/day with a taper; consider gastrointestinal and P. jirovecii prophylaxis |
| Grade 4 | Life-threatening; mechanical ventilation indicated | Permanent | Prednisone 1 - 2 mg/kg/day with a taper; consider gastrointestinal and P. jirovecii prophylaxis |
| Grade 5 | Death | N/A | N/A |
| No. | Author | Sex/age (years)/malignancy | Diagnosis; interval between treatment with pembrolizumab and irAE | Treatment | Outcome |
|---|---|---|---|---|---|
| NSCLC: non-small cell lung carcinoma; irAE: immune-related adverse event. | |||||
| 1 | Correia et al, 2022 [1] | Male/81/bladder cancer | Pruritus; 15 weeks | Pneumonitis: discontinuation of pembrolizumab | Bullous pemphigoid: improvement after 10 days |
| Pruritus/bullous pemphigoid: topical clobetasol, oral prednisolone, doxycycline, antihistamine | |||||
| Pneumonitis; 33 weeks | |||||
| Bullous pemphigoid; 36 weeks | |||||
| 2 | Cardona et al, 2021 [2] | Male/73/lung adenocarcinoma (NSCLC) | Pneumonitis (grade 2) | Adrenal insufficiency: corticosteroids | Pneumonitis, adrenal insufficiency: resolution |
| Adrenal insufficiency; 75 weeks (25 cycles) | Bullous pemphigoid: lack of response to corticosteroids and infliximab, resolution with cyclophosphamide | ||||
| Bullous pemphigoid; 2.5 years (35 cycles) | Bullous pemphigoid: intravenous methylprednisolone, infliximab, oral cyclophosphamide | ||||
| 3 | Alsabbagh et al, 2023 [3] | Male/68/bladder cancer | Hypertrophic lichenoid dermatitis; 3 months | Hypertrophic lichenoid dermatitis: topical mometasone | Hypertrophic lichenoid dermatitis: resolution |
| Bullous pemphigoid; 12 months | Bullous pemphigoid: transient discontinuation of pembrolizumab, oral prednisolone, and topical corticosteroids | Bullous pemphigoid: resolution within 2 months | |||
| Pneumonitis | Pneumonitis: permanent discontinuation of pembrolizumab | ||||
| 4 | Male/66/lung squamous cell carcinoma (non-small cell lung cancer) | Bullous pemphigoid; 16 months (21 cycles) | Bullous pemphigoid: topical clobetasol, oral bilastine | Bullous pemphigoid: improvement with topical corticosteroids, resolution with systemic corticosteroids within 2 weeks | |
| Pneumonitis (grade 3); 17 months (21 cycles) | Pneumonitis: discontinuation of pembrolizumab, intravenous methylprednisolone with proton pump inhibitor | Pneumonitis: gradual resolution within 2 weeks | |||