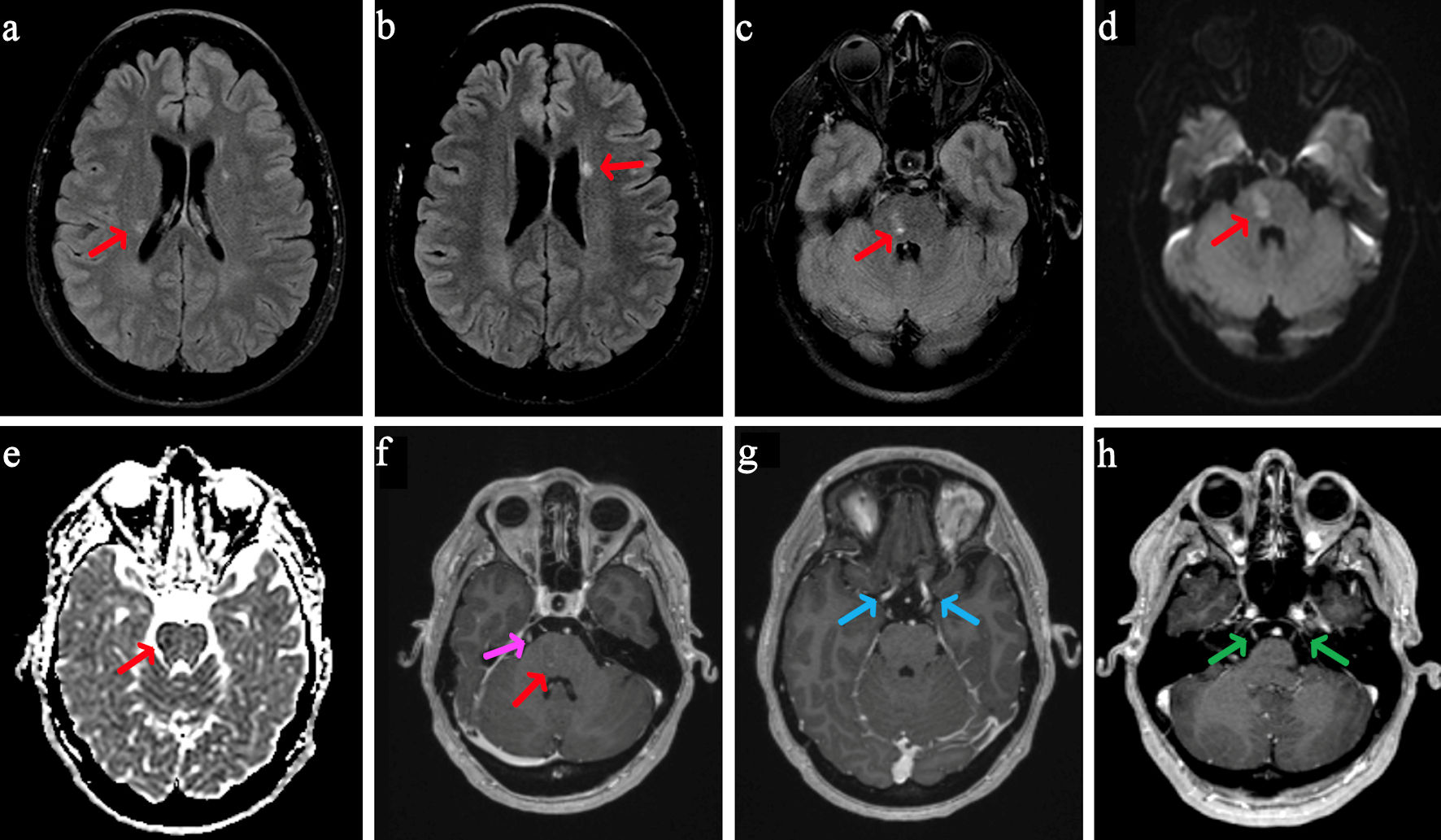
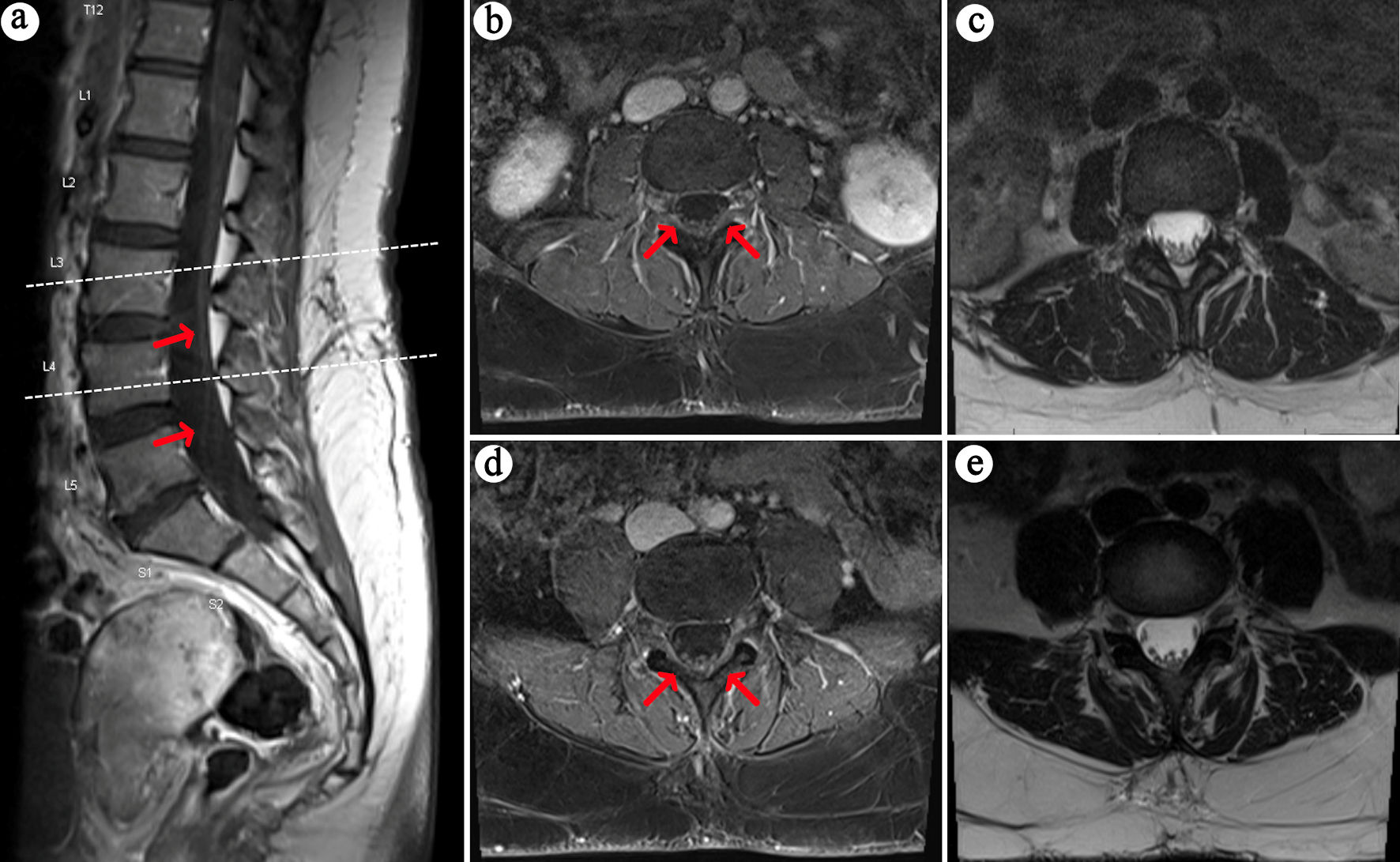
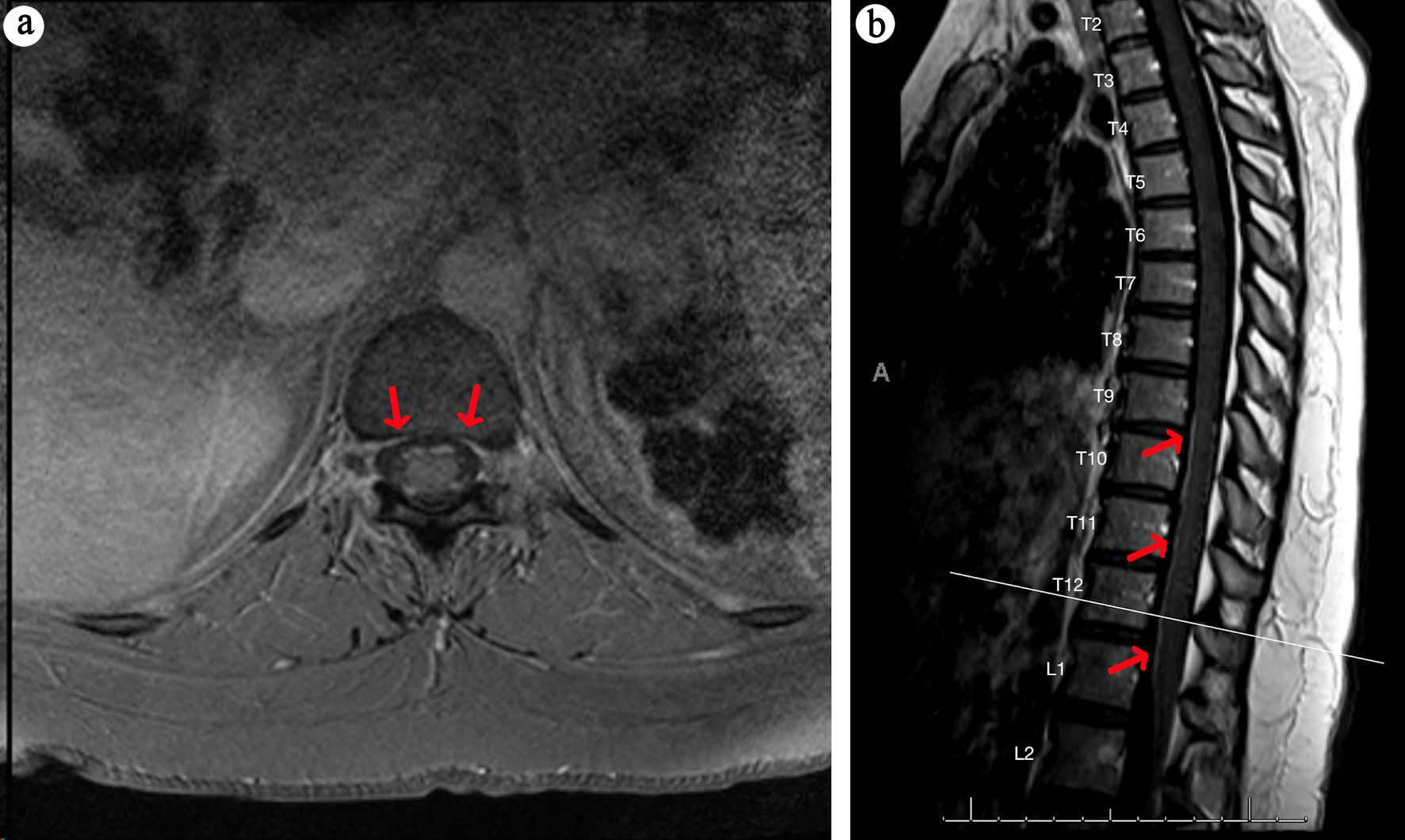
| Our case | F, 39 years | Patient presented with left face and hemibody paresthesias, abnormal gait with left leg dragging, weakness in left hip and knee, and absent BLE reflexes. | MS | Serum studies revealed a positive Sjogren’s anti-SSB antibody, despite no history of systemic or sicca symptoms. | MS and SS overlap | It is important to consider autoimmune overlap conditions in cases where there are atypical neurologic symptoms, as the co-occurrence of MS and SS can impact treatment strategies. |
| Mohamednour et al, 2020 [10] | F, 58 years | Patient with a 3-year history of MS presented with polyarthralgia, sicca symptoms, and Raynaud’s. | MS | Initial MRI of the brain and spine were reported as normal with few “nonspecific white matter areas”, complicating the diagnosis. | SS | CNS involvement in SS can closely resemble MS, complicating the diagnosis. Peripheral neuropathy and vasculitis are key differentiators. Early neurophysiological testing is crucial, and effective immunosuppression with shared decision-making can achieve successful outcomes. |
| Jung et al, 2000 [11] | F, 45 years | Patient with a 4-year history of dry eyes and mouth presented with BLE weakness, voiding difficulty, decreased visual acuity, BLE spasticity and hyperesthesia, and diffuse hyperreflexia. | MS | Erythematous skin lesions soon developed in the periauricular area and BLE. Skin and salivary gland biopsies both showed lymphocytic infiltration. | SS | SS should be considered as a differential diagnosis of a patient presenting with a syndrome that resembles MS because of the similarity in clinical manifestations and laboratory findings of both diseases. |
| Thong et al, 2002 [12] | F, 40 years | Patient with a history of a lacunar stroke with no residual deficits presented with acute urinary retention and diminished sensation below T5 spinal level, followed by a year of episodes of ataxia and upper limb dysmetria, retrobulbar neuritis, and pseudobulbar palsy. | UCTD | Five years after initial neurologic symptoms, the patient presented with pseudobulbar palsy and endorsed 1 year of sicca symptoms. Schirmer test was positive, and anti-Ro antibody was elevated. | SS | Patients with MS-like diseases should be carefully evaluated for other system autoimmune conditions as the treatment and prognosis differ from MS. Particularly, sicca symptoms should be sought as they often go unnoticed by patients. |
| De Santi et al, 2005 [13] | F, 48 years | Patient with a 29-year history of MS treated well with IFN-beta 1a for 5 years presented with xerophthalmia and xerostomia with dysphagia. | MS | N/A | MS and SS overlap | SS can develop long after the onset of MS, even during successful IFN-beta treatment. This case highlights the need to monitor MS patients for SS, as symptoms may arise unexpectedly, complicating diagnosis and management. |
| Guzel et al, 2022 [14] | F, 51 years | Patient with a history of PBC and Hashimoto thyroiditis presented with numbness and weakness in the left upper and lower extremities; further questioning revealed symptoms of dry eyes and mouth. | MS and SS overlap | N/A | MS and SS overlap | This case highlights the rare coexistence of four autoimmune diseases, emphasizing the importance of considering multiple overlapping autoimmune disorders in complex presentations. |
| Liu et al, 2014 [15] | F, 75 years | Patient presented with recurring numbness and sensory changes in BUE and BLE with zonesthesia in the waist. | MS | Patient had subsequent worsening of paresthesias and swelling of the parotid gland; oral and ocular sicca syndromes were suspected from history taking and confirmed by Schirmer test and labial salivary gland biopsies | SS | SS should be considered in the differential diagnosis of patients presenting with MS-like neurological symptoms, as it can mimic MS both clinically and neuroradiologically. |