Acute Myeloid Leukemia With a Non-Canonical FLT3 V491L Mutation: A Case Report With Ex Vivo FLT3 Inhibitors Sensitivity Testing
DOI:
https://doi.org/10.14740/jmc5197Keywords:
Acute myeloid leukemia, Gilteritinib, FLT3 V491L mutationAbstract
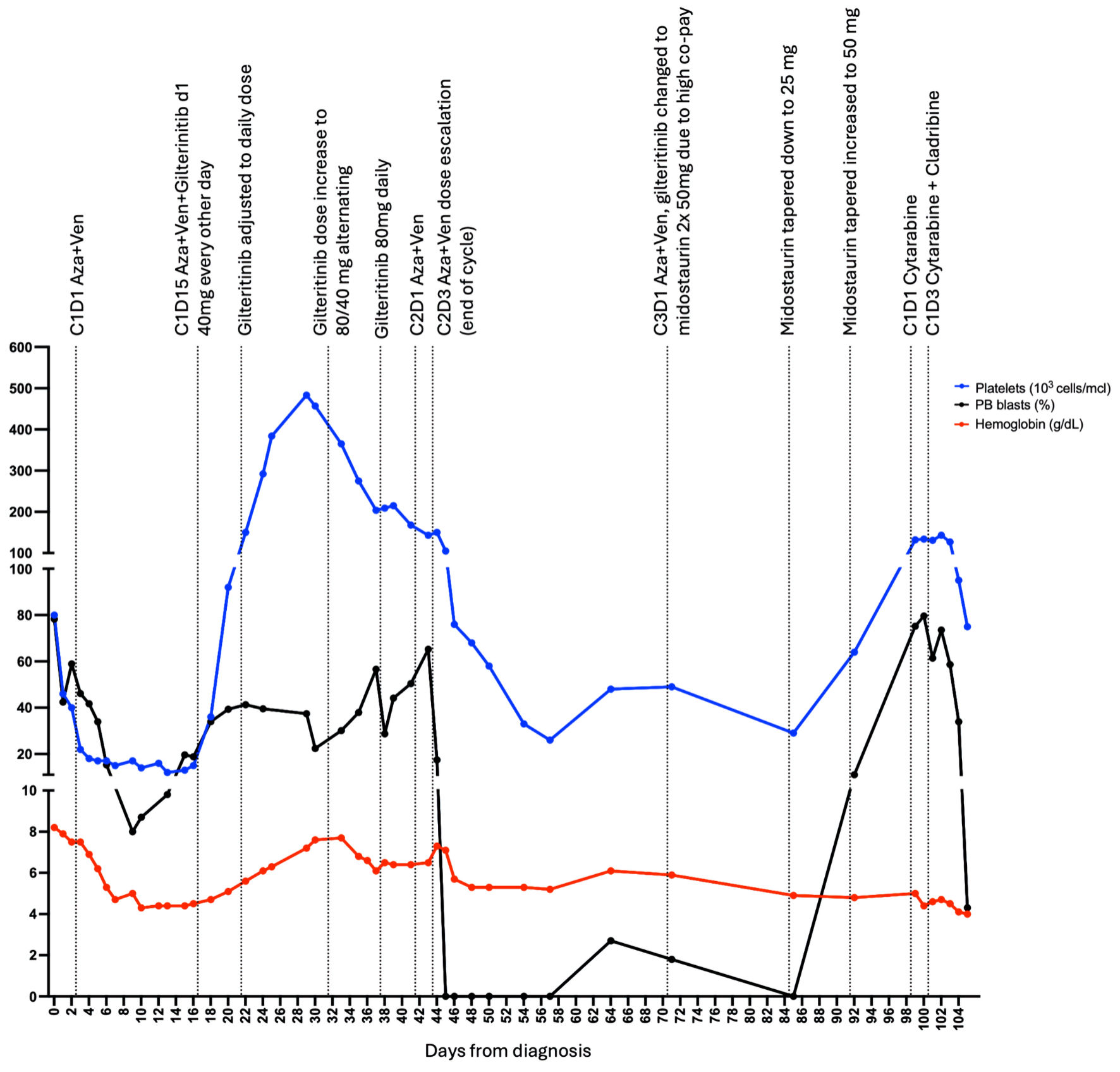
Approximately 30% of patients with acute myeloid leukemia (AML) harbor FMS-like tyrosine kinase 3 (FLT3) mutations, which are associated with poor overall survival. Although United States Food and Drug Administration (FDA)-approved FLT3 inhibitors are available, their efficacy against non-canonical FLT3 mutations remains elusive. Here we present a case of a 72-year-old female Jehovah’s Witness with newly diagnosed AML carrying a rare pathogenic FLT3 V491L mutation identified by next-generation sequencing. Given the patient’s religious beliefs, blood transfusion was not an option, making the patient ineligible for high-intensity chemotherapy and leading to alternative treatment approaches. To our knowledge, this is the first case report of the effectiveness of gilteritinib in an older patient with AML with a non-canonical FLT3 mutation and limitation on blood products usage. Initial treatment with hydroxyurea and leukapheresis followed by azacitidine and venetoclax resulted in an inadequate treatment response. Given the lack of research on the FLT3 V491L mutation, we conducted an ex vivo sensitivity study using the patient’s diagnostic bone marrow blasts to assess and compare the anti-leukemic efficacy of midostaurin, quizartinib, and gilteritinib. The ex vivo study revealed the lowest half-maximal inhibitory concentration (IC50) value and the highest number of apoptotic cells in gilteritinib treated patient’s blasts under Flt3 ligand-supplemented conditions. An initial clinical improvement with gilteritinib was observed. However, after the third cycle, gilteritinib was substituted with midostaurin because of high copay costs with gilteritinib. Subsequently, an increase in leukemic blasts was observed, and soon after, the patient expired. Treatment of relapsed AML with a non-canonical mutation is challenging due to the lack of data regarding FLT3 inhibitors. This case highlights the potential role of gilteritinib in targeting the rare FLT3 V491L mutation, underscoring the need for further research and improved accessibility to effective therapies.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution 4.0 International License.









