| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com |
Case Report
Volume 16, Number 8, August 2025, pages 309-313
Pure Red Cell Aplasia After ABO-Incompatible Allogeneic Hematopoietic Stem Cell Transplantation Successfully Treated With Daratumumab: Report of Two Cases
Emiliano Javier Bertonea, b, d, Martin Milanesioa, b, Mercedes de Jesus Garciaa, b, Leandro Roberto Castellanosa, b, Evelin Luciana Pirazzinib, c, Virginia Alicia Damonteb, c, Ana Lisa Basquieraa, b
aHematology and Oncology Service, Hospital Privado Universitario de Cordoba, Argentina
bInstituto Universitario de Ciencias Biomedicas de Cordoba, Argentina
cTransfusion Medicine Service, Hospital Privado Universitario de Cordoba, Argentina
dCorresponding Author: Emiliano Bertone, Hematology and Oncology Service, Hospital Privado Universitario de Cordoba, Argentina
Manuscript submitted June 14, 2025, accepted August 13, 2025, published online August 22, 2025
Short title: Daratumumab in Post-HSCT ABO-Incompatible PRCA
doi: https://doi.org/10.14740/jmc5154
| Abstract | ▴Top |
Pure red cell aplasia (PRCA) is a potential complication after ABO-incompatible allogeneic hematopoietic stem cell transplantation (HCT). In case where PRCA persists beyond 60 days post-HCT, spontaneous resolution is rare, and therapeutic intervention is typically required. However, there is currently no established standard of care for its management. We report two cases of post-transplant PRCA that were refractory to conventional therapies, including erythropoietin and rituximab, and were successfully treated with daratumumab. These cases underscore the potential role of daratumumab as an effective therapeutic option in the management of PRCA following ABO-incompatible HCT. Given the limited data available on its use in this setting, our report contributes with valuable clinical evidence supporting its efficacy and safety.
Keywords: Pure red cell aplasia; Allogenic hematopoietic stem cell transplant; Daratumumab
| Introduction | ▴Top |
Allogeneic hematopoietic stem cell transplantation (allo-HCT) is a potentially curative therapy for various hematologic disorders [1]. The ABO blood group system has been used in donor selection alongside other factors that influence HCT outcomes. Since human leukocyte antigen (HLA) and ABO blood group systems are inherited independently, ABO incompatibility has been reported in up to 50% of transplants, with studies showing contradictory effects on transplant survival [1-3]. Transplant complications observed in ABO-incompatible transplants include autoimmune hemolytic anemia (AIHA), pure red cell aplasia (PRCA), and, in rare cases, passenger lymphocyte syndrome [2]. PRCA typically arises in the context of major or bidirectional ABO incompatibility between donor and recipient, with an incidence ranging from 7.5% to 30% [4]. It is clinically characterized by anemia, reticulocytopenia, and red blood cell transfusions persisting for 30 to 90 days post-transplant. Bone marrow aspirates or biopsies reveal a marked reduction or complete absence of erythroid precursors [5]. The pathogenesis of PRCA is primarily attributed to the persistence of high titers of anti-donor isohemagglutinins targeting erythroid precursors. Conditioning regimens with reduced intensity or toxicity, such as fludarabine-busulfan, may predispose patients to PRCA due to the survival of recipient plasma cells which continue to produce isohemagglutinins [4, 6, 7]. Interestingly, the development of graft-versus-host disease (GVHD) appears to confer a protective effect against PRCA, likely through immune-mediated elimination of residual recipient plasma cells [8].
Therapeutic options described for post-transplant PRCA include: reduction of immunosuppression, plasmapheresis, high-dose erythropoietin (EPO), donor lymphocyte infusions (DLIs), anti-thymocyte globulin (ATG), rituximab, corticosteroids, and daratumumab, among others [9-12]. Daratumumab is a human IgG1κ monoclonal antibody directed against the CD38 antigen, which is highly expressed on plasma cells. The mechanisms of action of daratumumab include complement-dependent cytotoxicity, antibody-dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis, and apoptotic signaling [13]. In the context of PRCA following allo-HCT, cases successfully treated with daratumumab have been described [8, 9, 11, 14-17]. It has also been successfully used in the management of autoimmune hemolytic anemia (AIHA) [18] and Evans syndrome after HCT [19].
Our objective was to describe two cases of post-HCT PRCA successfully treated with daratumumab.
| Case Reports | ▴Top |
Case 1
A 46-year-old female was diagnosed with chronic neutrophilic leukemia with CSF3R mutation. First-line treatment was decided with HCT with an HLA-mismatched unrelated donor (9/10), with major ABO incompatibility (donor group B+/recipient O+). Conditioning was with fludarabine/busulfan, with intermediate intensity [20]. As prophylaxis for GVHD, she received tacrolimus, rabbit ATG, and methotrexate on days +1, +3, and +6. The infused cellularity was CD34+: 7.01 × 106/kg (peripheral blood). As an intercurrence, she presented febrile neutropenia, without microbiological isolation. Neutrophil engraftment occurred 14 days after transplantation, and the minimum platelet count during transplantation was 35,000 kJ/µL. The patient presented slow red blood cell (RBC) engraftment with persistent anemia with a low reticulocyte count and no signs of hemolysis, requiring a transfusion of two units of RBCs weekly to maintain hemoglobin levels above 7 g/dL, and was unresponsive to EPO treatment. No signs of acute GVHD were observed. Cytomegalovirus (CMV) viral load and a direct Coombs test were negative. Donor chimerism in whole blood at days +30 and +60 was 92% and 96%, respectively. Bone marrow biopsy revealed findings consistent with PRCA. Following the exclusion of secondary causes, parvovirus B19, CMV, and GVHD, a diagnosis of post-transplant PRCA was established. Due to the ongoing transfusion requirement, the patient’s ferritin level rose to 1989 ng/mL, and iron chelation with deferasirox was initiated. As transfusion dependence persisted beyond day +100, rituximab was administered at a dose of 375 mg/m2 weekly for four consecutive weeks. Given the lack of clinical response, daratumumab therapy was initiated 1 month after the final rituximab dose. The patient received four weekly intravenous infusions of daratumumab at 16 mg/kg, each preceded by premedication with intravenous dipyrone and hydrocortisone. Transfusion independence was achieved after the third dose of daratumumab (Table 1). At 755 days post-transplant, the patient remains in sustained hematological recovery, with no evidence of disease relapse or GVHD.
 Click to view | Table 1. Clinical Summary of Two Patients Receiving Post-Transplantation Daratumumab for Anemia-Associated ABO Major Incompatibility |
Case 2
A 58-year-old male was diagnosed with myelodysplastic syndrome with excess blasts 2, classified as high risk with a revised International Prognostic Scoring System (R-IPSS) score of 4.5. Following cytoreduction with five cycles of azacytidine, the patient underwent allo-HCT from a 10/10 HLA-matched unrelated donor. The stem cell source was bone marrow, and the transplant involved major ABO incompatibility (donor blood group A+, recipient O+). Conditioning was performed using an intermediate intensity fludarabine-busulfan regimen [20]. GVHD prophylaxis included methotrexate, tacrolimus, and rabbit ATG. The infused graft contained CD34+ cells at a dose of 4.18 × 106/kg (bone marrow). During the post-transplant period, the patient developed febrile neutropenia and was treated with piperacillin-tazobactam for 5 days. Neutrophil and platelet engraftment occurred on days +12 and +17 days, respectively. RBC engraftment was delayed, with persistent anemia, reticulocytopenia, and no evidence of hemolysis. Weekly transfusions of one RBC unit were required to maintain hemoglobin levels above 7 g/dL. The patient did not respond to EPO therapy. No signs of acute GVHD were observed. CMV viral load and direct Coombs test were negative. Donor chimerism at days +30 and +60 was 92% and 99%, respectively. Bone marrow biopsy findings were compatible with PRCA. In the context of anemia with reticulocytopenia and a bone marrow biopsy confirming PRCA, after excluding secondary causes (parvovirus B19, CMV, and GVHD), a diagnosis of post-transplant PRCA was made. As transfusion dependence persisted beyond day +100, rituximab was initiated at a dose of 375 mg/m2 weekly. The patient’s ferritin level was elevated at 3,665 ng/mL, and he exhibited intolerance to deferasirox. Due to ongoing transfusion requirements, daratumumab therapy was initiated 1 month after the final rituximab dose. The patient received four weekly intravenous infusions of daratumumab at 16 mg/kg, each preceded by premedication with intravenous dipyrone and hydrocortisone. Transfusion independence occurred 15 days after initiating daratumumab (Fig. 1, Table 1). Ferritin levels progressively declined, until reaching 679 ng/mL at last follow-up. At 1,180 days post-transplant, the patient remains with sustained hematological recovery, with no evidence of disease relapse or GVHD.
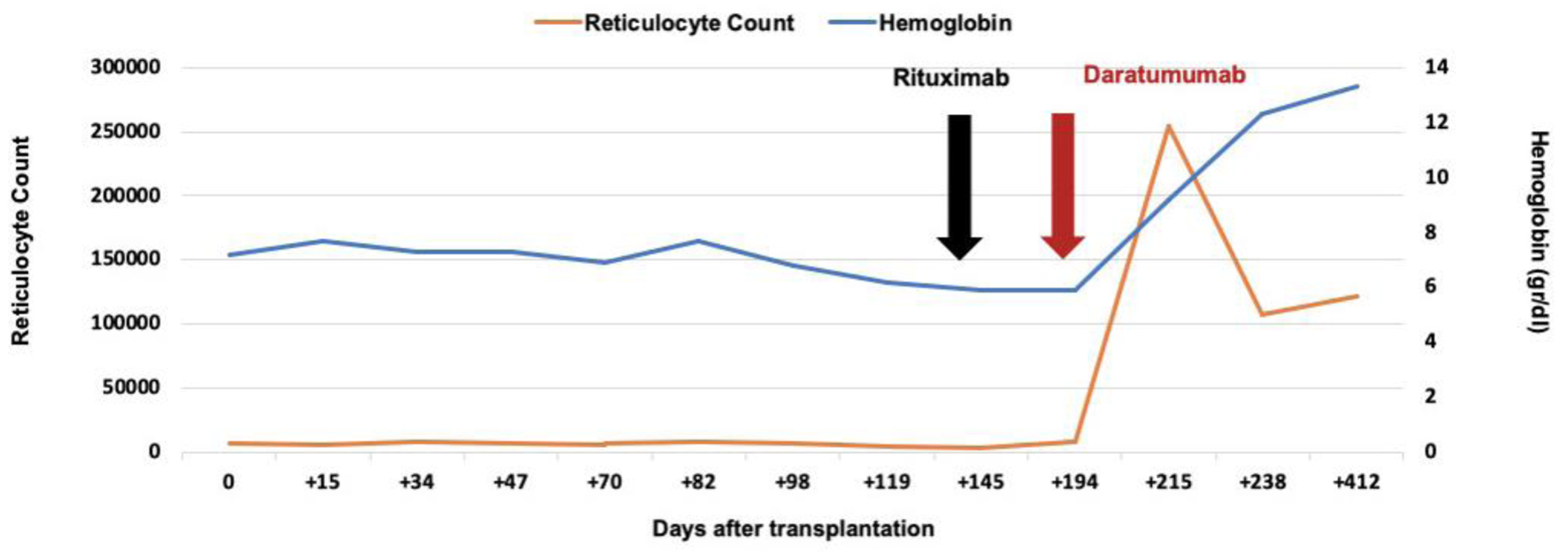 Click for large image | Figure 1. Curve of hemoglobin and reticulocytes values, and response to treatment with daratumumab. Changes in hemoglobin concentration (blue line, right y-axis) and absolute reticulocyte count (orange line, left y-axis) in a patient after allogeneic hematopoietic cell transplantation with ABO major incompatibility (case 2). The x-axis indicates days after transplantation. The black arrow marks the initiation of rituximab treatment, and the red arrow marks the initiation of daratumumab treatment. A marked increase in reticulocyte count and gradual recovery of hemoglobin were observed following daratumumab administration. |
| Discussion | ▴Top |
We reported two cases of PRCA following allo-HCT. Both patients underwent a major ABO-incompatible transplant, received a busulfan-based, reduced-toxicity, intermediate-intensity conditioning regimen, and they did not develop GVHD after transplant, illustrating key risk factors associated with PRCA in the post-transplant setting. After a prolonged period of transfusion dependence, both patients achieved successful erythroid recovery after treatment with daratumumab.
When PRCA persists beyond 60 days after HCT, spontaneous resolution is uncommon, and therapeutic intervention is generally warranted due to the significant morbidity associated with transfusion dependence [17]. Currently, there is no approved standard of care for its management [12]. Although EPO has shown benefit in select cases, its use is not supported as a standard treatment due to inconsistent responses and the lack of consensus in the literature [3]. Similarly, rituximab and DLI have demonstrated limited efficacy in this context [3].
Considering the potential of daratumumab to eliminate the recipient residual plasma cells, cases of post-transplant PRCA were reported since 2018. A systematic review of seven studies involving 28 patients treated with daratumumab for post-HCT PRCA reported an overall response rate of 85%, with 20 patients achieving a complete response (CR) and four patients achieving partial response (PR). The median time to response was 4 weeks (range: 2 - 8 weeks). Among those who achieved CR, 17 maintained their response at a median follow-up of 12 months. The most frequently reported adverse event was neutropenia, ocurring in 35% of cases [21]. Weverling et al [22] described 14 patients with PRCA after ABO-incompatible allo-HCT treated with daratumumab in seven Dutch bone marrow transplant centers. Transfusion independence was achieved in 93% of patients, with a median time to response of 14 days. No unexpected adverse events or relapses were reported at a median follow-up of 383 days [22]. Consistent with these findings, our case report further supports the rapid and sustained efficacy of daratumumab in resolving PRCA.
Neither of our patients experienced adverse events during daratumumab treatment. However, a recent multicenter study involving 45 patients with post-transplant PRCA [23] reported serious adverse events, primarily infections, in seven patients treated with daratumumab. In that study, daratumumab was administered either as first-line (36%) or in subsequent lines, with a median time from PRCA diagnosis to treatment initiation of 88 days. Infusion schedules and total doses varied (range 1 - 8), and no correlation between treatment regimen and adverse events was informed [23].
Our two cases received four doses of daratumumab, with one patient showing response after the second dose. This may suggest that a limited course of daratumumab can achieve durable remission with a favorable safety profile. To limit the number of infusions until the development of reticulocytosis and normalization of the ABO-titer has been proposed with the potential of reduce adverse events [22].
Our two patients received daratumumab after +194 and +266 days after transplant, showing by this point a significant iron overload after a large number of transfusions. This highlights the potential role of this therapy earlier in the course of PRCA development. The accumulating evidence from case series, systematic reviews, and now larger real-world cohorts reinforces the role of daratumumab as an effective and well-tolerated option for PRCA following allo-HCT.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors have no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Conceptualization: EJB and ALB; methodology: EJB, ALB, MM, and LRC; resources: EJB, ALB, and MJG; visualization (tables and figures): VAD and ELP; writing - original draft preparation: EJB, ALB, MM, and LRC; writing - review and editing: EJB, ALB, ELP, and VAD; supervision: ALB, ELP, and VAD; project administration: ALB and EJB. All authors have read and agreed to the published version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
AIHA: autoimmune hemolytic anemia; ATG: anti-thymocyte globulin; CMV: cytomegalovirus; DLI: donor lymphocyte infusion; EPO: erythropoietin; GVHD: graft-versus-host disease; HCT: hematopoietic stem cell transplantation; MDS: myelodysplastic syndrome; PCR: polymerase chain reaction; PR: partial response; PRCA: pure red cell aplasia; CR: complete response; RCB: red blood cell; R-IPSS: revised International Prognostic Scoring System; TCI: transplant conditioning intensity
| References | ▴Top |
- Guru Murthy GS, Logan BR, Bo-Subait S, Beitinjaneh A, Devine S, Farhadfar N, Gowda L, et al. Association of ABO mismatch with the outcomes of allogeneic hematopoietic cell transplantation for acute leukemia. Am J Hematol. 2023;98(4):608-619.
doi pubmed - Guven M, Peczynski C, Boreland W, Blaise D, Peffault de Latour R, Yakoub-Agha I, Gedde-Dahl T, et al. The impact of ABO compatibility on allogeneic hematopoietic cell transplantation outcomes: a contemporary and comprehensive study from the transplant complications working party of the EBMT. Bone Marrow Transplant. 2025;60(7):956-963.
doi pubmed - Longval T, Galimard JE, Lepretre AC, Suarez F, Amiranoff D, Cazaux M, Kaphan E, et al. Treatment for pure red cell aplasia after major ABO-incompatible allogeneic stem cell transplantation: a multicentre study. Br J Haematol. 2021;193(4):814-826.
doi pubmed - Aung FM, Lichtiger B, Bassett R, Liu P, Alousi A, Bashier Q, Ciurea SO, et al. Incidence and natural history of pure red cell aplasia in major ABO-mismatched haematopoietic cell transplantation. Br J Haematol. 2013;160(6):798-805.
doi pubmed - Zhu KE, Li JP, Zhang T, Zhong J, Chen J. Clinical features and risk factors of pure red cell aplasia following major ABO-incompatible allogeneic hematopoietic stem cell transplantation. Hematology. 2007;12(2):117-121.
doi pubmed - Griffith LM, McCoy JP, Jr., Bolan CD, Stroncek DF, Pickett AC, Linton GF, Lundqvist A, et al. Persistence of recipient plasma cells and anti-donor isohaemagglutinins in patients with delayed donor erythropoiesis after major ABO incompatible non-myeloablative haematopoietic cell transplantation. Br J Haematol. 2005;128(5):668-675.
doi pubmed - Kopko PM. Transfusion support for ABO-incompatible progenitor cell transplantation. Transfus Med Hemother. 2016;43(1):13-18.
doi pubmed - Jeyaraman P, Borah P, Rajput P, Dayal N, Pathak S, Naithani R. Daratumumab for pure red cell aplasia post ABO incompatible allogeneic hematopoietic stem cell transplant for aplastic anemia. Blood Cells Mol Dis. 2021;88:102464.
doi pubmed - Wu C, Manchen P, Edelman A, Husnain M, Katsanis E, Fuchs D, Stephens L, et al. Refractory pure red blood cell aplasia secondary to major ABO-incompatible allogeneic stem cell transplantation successfully treated with daratumumab. J Hematol. 2023;12(6):277-282.
doi pubmed - Tsai HJ, Lin SF, Liu TC, Chang CS, Hsiao HH, Chen TP. Pure red cell aplasia after ABO major-mismatched allogeneic peripheral blood stem cell transplantation successfully treated with plasma exchange and low-dose steroid: two case reports. Kaohsiung J Med Sci. 2004;20(3):128-132.
doi pubmed - Bathini S, Holtzman NG, Koka R, Singh Z, Wilding E, Zou Y, Ruehle K, et al. Refractory postallogeneic stem cell transplant pure red cell aplasia in remission after treatment with daratumumab. Am J Hematol. 2019;94(8):E216-E219.
doi pubmed - Marco-Ayala J, Gomez-Segui I, Sanz G, Solves P. Pure red cell aplasia after major or bidirectional ABO incompatible hematopoietic stem cell transplantation: to treat or not to treat, that is the question. Bone Marrow Transplant. 2021;56(4):769-778.
doi pubmed - de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, Oomen LA, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840-1848.
doi pubmed - Dovern E, Biemond BJ, Nur E. Case report: Successful treatment with daratumumab for pure red cell aplasia in a patient with mixed lymphoid chimerism after ABO-mismatched stem cell transplant for sickle cell disease. Front Immunol. 2023;14:1212007.
doi pubmed - Metafuni E, Busnego Barreto MT, Valentini CG, Giammarco S, Limongiello MA, Sora F, Bianchi M, et al. Pure red cell aplasia among ABO mismatched hematopoietic stem cell transplant recipients: a 13-years retrospective study and literature review. Front Oncol. 2024;14:1386670.
doi pubmed - Chapuy CI, Kaufman RM, Alyea EP, Connors JM. Daratumumab for Delayed Red-Cell Engraftment after Allogeneic Transplantation. N Engl J Med. 2018;379(19):1846-1850.
doi pubmed - Martino R, Garcia-Cadenas I, Esquirol A. Daratumumab may be the most effective treatment for post-engraftment pure red cell aplasia due to persistent anti-donor isohemagglutinins after major ABO-mismatched allogeneic transplantation. Bone Marrow Transplant. 2022;57(2):282-285.
doi pubmed - Frioni F, Metafuni E, Limongiello MA, Piccirillo N, Massini G, Pellegrino C, Giammarco S, et al. Posttransplant autoimmune hemolytic anemia with anti-D specificity successfully treated with daratumumab: a case report. Transfus Med Hemother. 2024;51(5):355-358.
doi pubmed - Blennerhassett R, Sudini L, Gottlieb D, Bhattacharyya A. Post-allogeneic transplant Evans syndrome successfully treated with daratumumab. Br J Haematol. 2019;187(2):e48-e51.
doi pubmed - Spyridonidis A, Labopin M, Savani BN, Niittyvuopio R, Blaise D, Craddock C, Socie G, et al. Redefining and measuring transplant conditioning intensity in current era: a study in acute myeloid leukemia patients. Bone Marrow Transplant. 2020;55(6):1114-1125.
doi pubmed - Basharat A, et al. AML-214 the role of daratumumab in treating pure red cell aplasia after allogeneic transplant in patients with ABO incompatibility: a systematic review. Clinical Lymphoma Myeloma and Leukemia. 2024;24:S300-S301.
- Weverling F, Roeven M, Nijssen C, Broers AEC, Dovern E, van Rhenen A, Sluis GV, et al. Efficacy and safety of daratumumab in pure red cell aplasia after allogeneic transplantation: Dutch real-world data. Blood Adv. 2024;8(7):1683-1686.
doi pubmed - Gagelmann N, Witte M, Peczynski C, Boreland W, Broers AEC, Jost E, Kulagin A, et al. Daratumumab for PRCA after HCT: study and practical considerations from the EBMT Transplant Complications Working Party. Blood Cancer J. 2025;15(1):106.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.