| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com |
Case Report
Volume 16, Number 5, May 2025, pages 159-163
Lacosamide-Induced Complete Heart Block in a Healthy Young Female
Kai Shiang Lina, Jensen Georgea, Nazeera Ghaniea, Harshith Chandrakumara, Joseph Jayaraja, Asher Gorantlaa, Sabu Johna, b, Adam S. Budzikowskia
aSUNY Downstate Health Sciences University, Brooklyn, NY, USA
bCorresponding Author: Sabu John, SUNY Downstate Health Sciences University, Brooklyn, NY 11203, USA
Manuscript submitted March 27, 2025, accepted May 6, 2025, published online May 13, 2025
Short title: LCM-Induced CHB
doi: https://doi.org/10.14740/jmc4350
| Abstract | ▴Top |
New-onset complete heart block (CHB) occurring in a healthy young female is a relatively rare finding. We report a case of a 33-year-old female who was incidentally found to have CHB after presenting with a left tibial shaft fracture after a fall. Despite a past medical history of epilepsy, the maintenance of a long, seizure-free period coupled with the absence of neuromuscular weakness prompted a thorough investigation, and the etiology of the CHB was ultimately determined to be lacosamide (LCM), the antiseizure medication. The discontinuation of LCM resulted in spontaneous conversion to sinus rhythm, and an outpatient follow-up 4 weeks after discharge revealed no further episodes of falls, heart block, or other symptoms. We believe this is a rare presentation of LCM-induced CHB in an otherwise healthy young female.
Keywords: Lacosamide; Heart block; Seizure; Epilepsy; Structural heart disease
| Introduction | ▴Top |
Complete heart block (CHB) is a pathological heart rhythm, in which there is an absence of electrical conduction through the atrioventricular (AV) node, resulting in complete dissociation of the atria and ventricles [1]. The defect in the cardiac conduction system can occur at any location between the AV node and the Purkinje fibers, and typically causes characteristic findings on electrocardiography (ECG) such as a regular P-P interval, an absent relationship between the P waves and QRS complexes, and the presence of more P waves than QRS complexes. Although any degree of AV block is common, third-degree AV block is rare, with an incidence of approximately 0.02% in the general population.
Lacosamide (LCM), an antiepileptic agent that selectively enhances the slow inactivation of voltage-gated sodium channels, exerts its antiseizure effects through hyperexcitable neuronal membranes and inhibition of repetitive neuronal firing [2]. It is Food and Drug Administration (FDA)-approved for both monotherapy and adjunctive therapy in partial-onset seizures and for adjunctive therapy only in primary generalized tonic-clonic seizures. LCM is usually well-tolerated and common side effects include dizziness, nausea, vertigo, abnormal coordination, and ataxia [3].
In this report, we present a rare case of LCM-induced CHB in a patient presenting with a left tibial shaft fracture after a fall. We highlight the clinical presentation, ECG findings, diagnostic approach, and management in this unique scenario.
| Case Report | ▴Top |
Investigations
A 33-year-old female with a past medical history of epilepsy, cerebral palsy, and intellectual disability presented to the emergency department for left lower extremity pain after an unwitnessed fall. The morning prior to presentation, the patient fell after using the restroom and reportedly hit her left knee. She was unsure whether she had lost consciousness prior to the fall but was found on the ground by her mother. She denied any other trauma or nausea, warmth, or diaphoresis before the fall as well as any reported tongue biting, bowel or bladder incontinence, extremity weakness, or delayed return to baseline mental status according to her mother. She has been unable to ambulate due to pain. She has had no prior history of falls, and a review of systems was negative for dizziness, headache, chest pain, shortness of breath, palpitations, abdominal pain, bowel or bladder changes, or any constitutional symptoms. Her last seizure episode occurred over a year ago.
The patient did not smoke, drink, or use illicit or recreational drugs, and has always lived with her mother, who takes care of her 24 h a day, 7 days a week. The only recent travel they had was to Central America approximately 5 months before presentation.
Diagnosis and treatment
On arrival, she was afebrile, blood pressure (BP) of 110/68 mm Hg, a heart rate of 88 beats per minute, a respiratory rate of 20 breaths per minute, and an oxygen saturation of 98% as measured by pulse oximetry. Physical exam was notable for edema, erythema, and tenderness to the left ankle and tibia, and laboratory findings showed a serum creatinine level of 2.57 mg/dL, a serum potassium level of 5.6 mEq/L, a serum glucose of 168 mg/dL, a thyroid-stimulating hormone level of 4.67 µIU/mL, a normal free T4 level of 1.02 ng/dL, and a normal urine toxicology screen. An X-ray of the left lower extremity revealed a closed left spiral tibial shaft fracture with medial translation of the distal fragment (Fig. 1), and an external reduction and splinting was performed by the orthopedic team in the emergency department. The patient was admitted and planned for orthopedic surgery that same day, but the surgery was deferred owing to a preoperative ECG showing a junctional escape rhythm with a heart rate in the 30s in the setting of a CHB (Fig. 2). Soon after this ECG was taken, the patient had complained of dizziness and nausea, and had an episode of non-bilious, non-bloody vomiting. Because the patient failed to respond to initial treatment of acute hyperkalemia with calcium gluconate, insulin, and albuterol, the cardiology service was consulted and a transvenous pacemaker (TVP) was placed (Fig. 3). The patient was transferred to the cardiac care unit for further management of new-onset CHB with a potential need for permanent pacing.
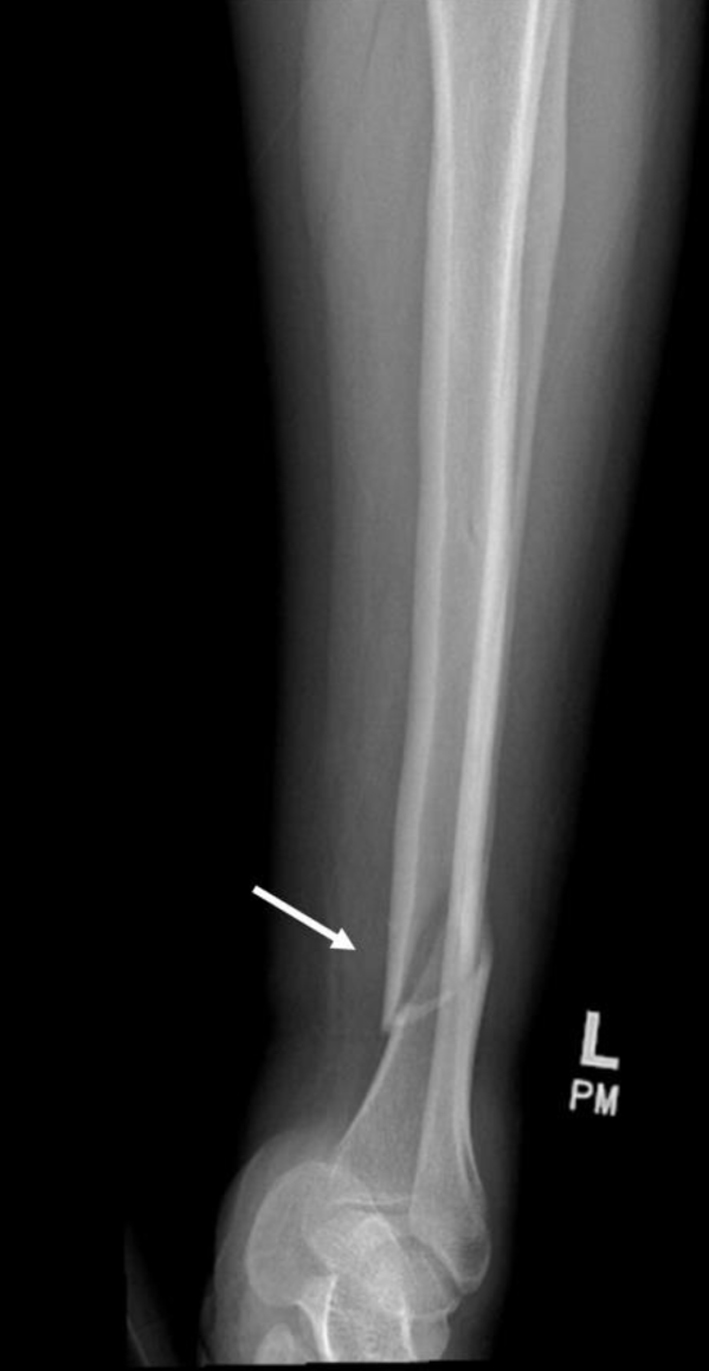 Click for large image | Figure 1. Left lower extremity X-ray showing closed left spiral tibial shaft fracture with medial translation of the distal fragment (arrow). |
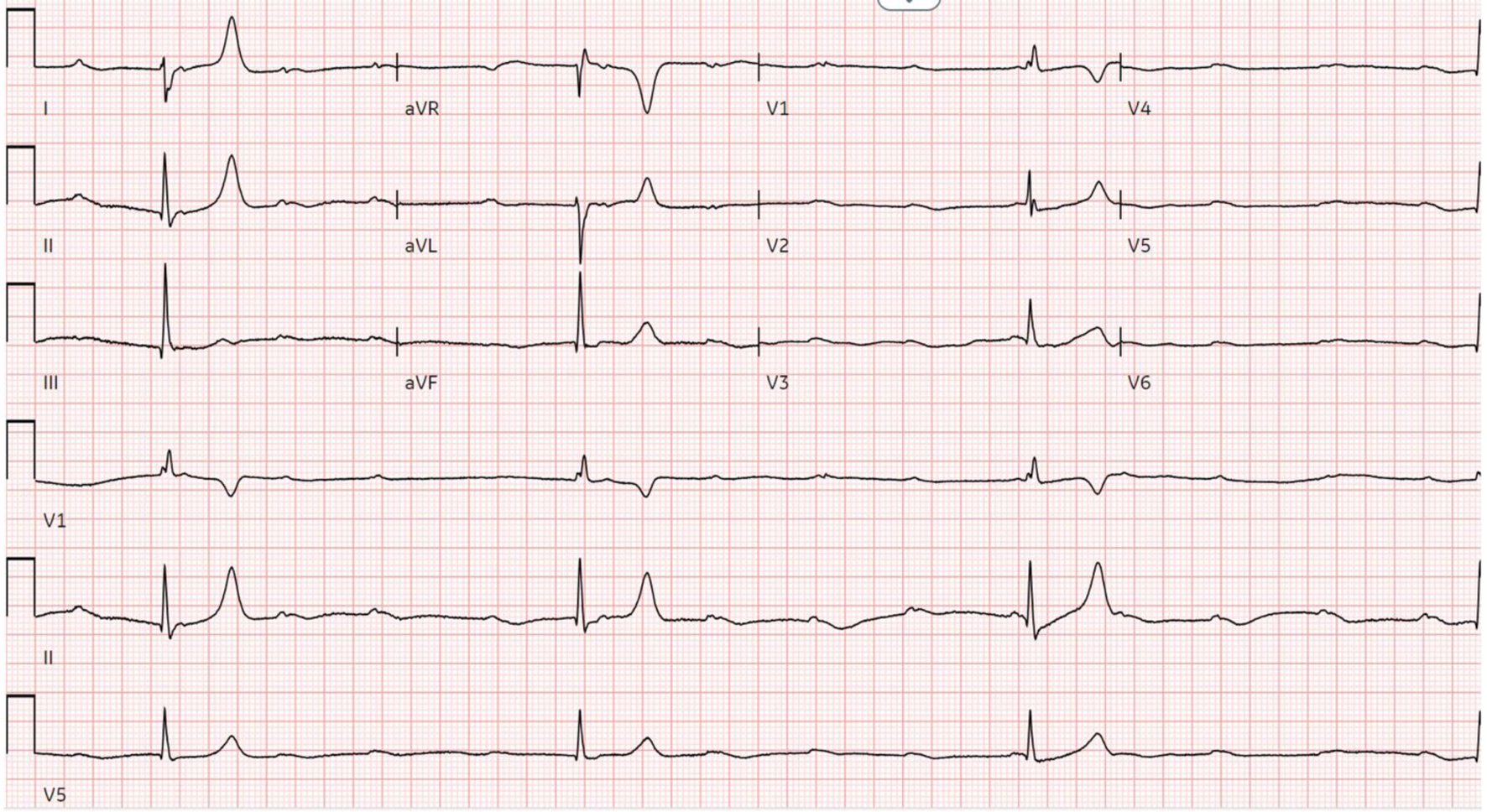 Click for large image | Figure 2. Preoperative ECG showing ventricular escape rhythm in the setting of third-degree AV block. AV: atrioventricular; ECG: electrocardiogram. |
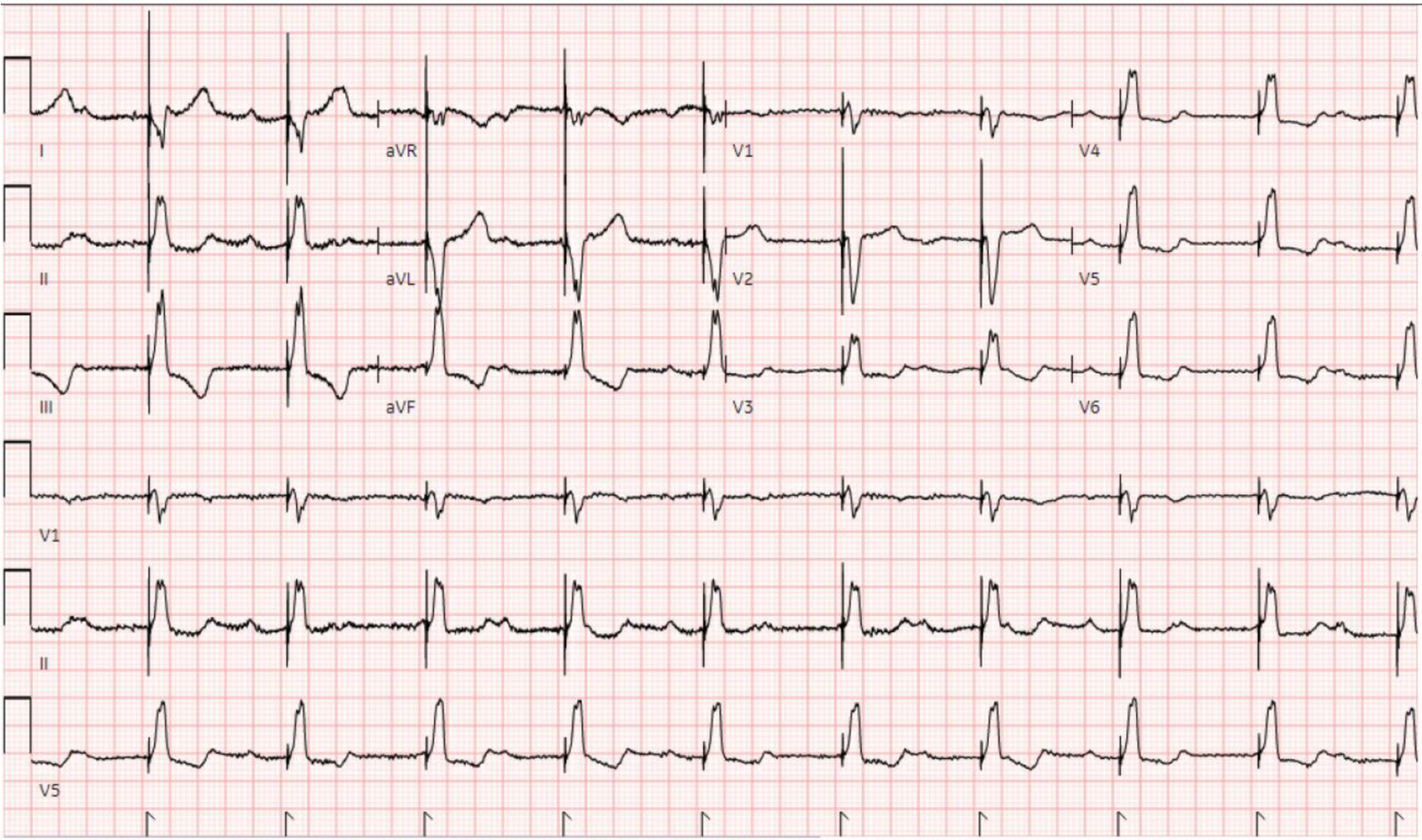 Click for large image | Figure 3. ECG after TVP placement showing a ventricular-paced rhythm. ECG: electrocardiogram; TVP: transvenous pacemaker. |
Home medications included lamotrigine, LCM, and levetiracetam.
After admission, a workup for reversible etiologies of CHB was begun. Erythrocyte sedimentation rate (ESR) was 54 mm/h and C-reactive protein (CRP) levels was 74 mg/dL, and the rest of the workup, including Lyme titers, human immunodeficiency virus (HIV) testing, tuberculosis (TB) testing, rapid plasma reagin (RPR), antinuclear antibodies (ANAs), extractable nuclear antigen antibodies (ENAs) panel, angiotensin-converting enzyme (ACE) levels, digoxin levels, and peripheral smear for parasitic protozoans, were within normal limits. A transthoracic echocardiogram (TTE) showed a grossly normal heart and function without valvular abnormalities or any congenital heart defects, and a 24-h video electroencephalogram (vEEG) was unremarkable for any breakthrough seizures, although it did show generalized cortical irritability with frontal predominance suggestive of generalized epilepsy syndrome.
On day 5 of admission, 3 days after LCM was switched to clobazam (as we considered LCM as a possible culprit), spontaneous resumption of AV nodal conduction was noted (Fig. 4). The patient eventually underwent open reduction and internal fixation of her left lower extremity and was discharged with clobazam in lieu of LCM.
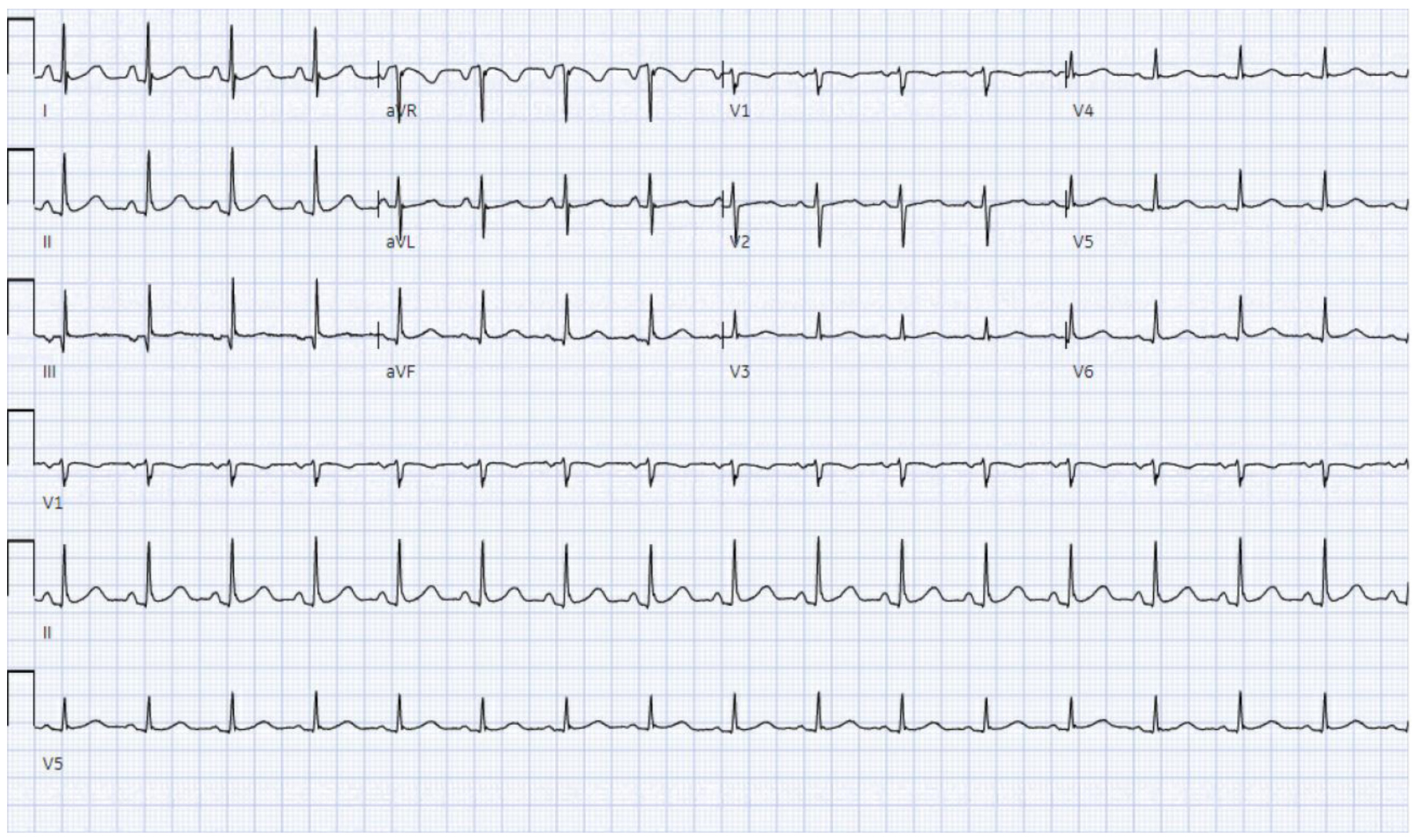 Click for large image | Figure 4. ECG demonstrating spontaneous reversion to sinus rhythm on the third day of LCM discontinuation. ECG: electrocardiogram; LCM: lacosamide. |
Follow-up and outcomes
At an outpatient follow-up visit 4 weeks after discharge, the patient continued to be in sinus rhythm and was asymptomatic with no further episodes of bradyarrhythmias or falls.
| Discussion | ▴Top |
The development of CHB at a young age of 33 years is somewhat unusual. In contrast to the idiopathic and fibrotic degeneration of the cardiac conduction system frequently presumed to be the etiology in older patients, reversible etiologies are more likely in the younger population, particularly in the absence of primary cardiac disease. In this patient who presented with a fracture after a fall, the etiology for her presentation was initially challenging to identify due to the absence of any prior history of falls, focal neurologic deficits, musculoskeletal degeneration, or any other known precipitating factor other than epilepsy, which had been well controlled. Although a seizure episode may have led to her fall, her compliance with a robust antiepileptic regimen, the absence of any recent breakthrough seizures, and an unremarkable vEEG suggest that seizures are less likely, prompting investigation into other etiologies. The incidental finding of CHB on preoperative ECG was fortuitous in that it not only provided an important diagnostic clue, but also led to her appropriate placement in the cardiac care unit for further management of the CHB. The development of CHB in unit without telemetry, where she would have been placed had the CHB not been detected prior to admission, during surgery, or even during the postoperative period could have been outcome-altering. We postulate that the fall was likely due to a syncopal or presyncopal episode in the setting of perhaps transient CHB.
In higher degrees of AV block, such as Mobitz type 2, high-grade AV block, or CHB, the diagnostic and management approach typically involve a search for reversible etiologies and if none, placement of a permanent pacemaker. Reversible etiologies for AV block are numerous and include structural heart disease, myocardial ischemia, myocarditis, endocarditis with abscess formation, electrolyte disturbances, hypothyroidism, medications such as AV nodal blocking agents, and post-procedural causes such as after cardiac surgery, catheter ablation, or transcatheter aortic valve implantation [4]. In more than half the cases, however, no reversible etiology is identifiable, and the block is considered idiopathic. In our patient, an infectious workup was negative for Lyme disease, HIV, TB, syphilis, and Chagas disease. Autoimmune etiologies were also less likely given the normal ANA and ENA panels. Structural heart disease and congenital heart defects were absent, and etiologies such as sarcoidosis, digoxin toxicity, myocardial ischemia, and myocarditis were also less likely due to normal ACE and digoxin levels, the absence of symptoms suggestive of acute or chronic coronary syndromes, and the clear hemodynamic stability that was maintained throughout hospitalization after placement of the TVP. Medication-related etiologies were also initially unlikely, despite temporarily discontinuing LCM, until spontaneous resumption of AV conduction was noted 3 days after drug cessation. Medications that cause CHB, or any degree of heart block, are usually those that prolong the AV nodal refractory period such as beta-blockers and calcium channel blockers. However, given the scarce reports of LCM causing AV block and the absence of a more likely etiology to explain the CHB, LCM was temporarily discontinued, a decision supported by the patient’s relatively low risk of developing breakthrough seizures due to an unremarkable vEEG and the maintenance of a seizure-free state for over a year. Rare reports of LCM causing conduction abnormalities described dose-dependent PR interval prolongation, symptomatic second-degree AV block, and second-degree AV block resulting in cardiac arrest, although these occurrences were noted in patients with pre-existing conduction disease, those who had received LCM concurrently with carbamazepine, and in neonates, respectively [5, 6]. There are also several reports describing LCM’s association with syncope, but they detailed patients who were either receiving LCM for diabetic neuropathy or those with cardiac risk factors and concomitant use of AV nodal blocking agents. Despite none of the clinical scenarios snugly fitting our patient, they do support the possibility of LCM causing bradyarrhythmias, such as CHB, in our patient. Another feature that strongly supports LCM causing CHB is the temporal relationship between LCM cessation and the resumption of normal AV conduction. The half-life of LCM is approximately 13 h and its duration of action ranges from 52 to 65 h, based on 4 or 5 half-lives, coinciding with the reversion to sinus rhythm that occurred on day 3 of LCM cessation, which fell within the 48- to 72-h time window.
Discontinuation of reversible etiologies for CHB typically results in resumption, as was the case seen in our patient, eliminating the need for permanent pacemaker implantation. At a follow-up visit 4 weeks after discharge, the patient continued to be in sinus rhythm and the absence of recurrent episodes cemented the diagnosis of LCM-induced CHB.
Conclusion
We report the case of a young female who presented to the hospital for a left lower extremity fracture after a fall. After extensive workup, the etiology of her fall was determined to be arrhythmogenic syncope secondary to CHB and discontinuation of the causative agent, LCM, resulted in the spontaneous resumption of AV conduction within 3 days. To the best of our knowledge, LCM-induced CHB is a are complication in an otherwise healthy young patient without cardiac comorbidities and adds to the sparse literature of LCM-associated conduction abnormalities. We aim to raise awareness of LCM-related arrhythmias, especially in the epileptic population.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the patient.
Author Contributions
The case was identified by supervising author Sabu John, MD. The manuscript was written by Kai Shiang Lin, MD. Manuscript editing was performed by Kai Shiang Lin, MD, Jensen George, MD, Nazeera Ghanie, MD, Harshith Chandrakumar, MD, Joseph Jayaraj, MD, Asher Gorantla, MD, Sabu John, MD, Adam S. Budzikowski, MD, PhD. The electrocardiogram and coronary angiogram image were identified by Sabu John, MD. The final manuscript was reviewed and supervised by Adam S. Budzikowski, MD, PhD.
Data Availability
The authors declare that data supporting the findings of this study are available within the article
Abbreviations
ACE: angiotensin-converting enzyme; ANAs: antinuclear antibodies; AV: atrioventricular; BP: blood pressure; CHB: complete heart block; CRP: C-reactive protein; ECG: electrocardiogram; ENA: extractable nuclear antigen; ESR: erythrocyte sedimentation rate; FDA: Food and Drug Administration; HIV: human immunodeficiency virus; LCM: lacosamide; mm Hg: millimeters of mercury; RPR: rapid plasma reagin; TB: tuberculosis; TTE: transthoracic echocardiogram; TVP: transvenous pacemaker; vEEG: video electroencephalogram
| References | ▴Top |
- Narula OS, Scherlag BJ, Javier RP, Hildner FJ, Samet P. Analysis of the A-V conduction defect in complete heart block utilizing His bundle electrograms. Circulation. 1970;41(3):437-448.
doi pubmed - Perucca E, Yasothan U, Clincke G, Kirkpatrick P. Lacosamide. Nat Rev Drug Discov. 2008;7(12):973-974.
doi pubmed - Ben-Menachem E, Biton V, Jatuzis D, Abou-Khalil B, Doty P, Rudd GD. Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia. 2007;48(7):1308-1317.
doi pubmed - Kashou AH, Goyal A, Nguyen T, Ahmed I, Chhabra L. Atrioventricular block. In: StatPearls. Treasure Island (FL) ineligible companies. 2025.
pubmed - Huberman MA, Mallar C, Kalika PM. Neonatal cardiac arrest following lacosamide treatment: a case report. Pediatr Neurol. 2023;149:184-186.
doi pubmed - Nizam A, Mylavarapu K, Thomas D, Briskin K, Wu B, Saluja D, Wong S. Lacosamide-induced second-degree atrioventricular block in a patient with partial epilepsy. Epilepsia. 2011;52(10):e153-155.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.