| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com |
Case Report
Volume 16, Number 6, June 2025, pages 212-221
Long Durable Response With Trastuzumab Deruxtecan Monotherapy in a Triple-Negative Metastatic Breast Cancer Patient With Human Epidermal Growth Factor Receptor 2 Mutation: A Long-Term Follow-Up and Literature Review
Muralidhar Idamakantia, e , Rani Indrani Bijjamb
, Manoj Kumarc, Shiva Kumar Mukkamallad
aAdult Inpatient Medical Services (AIMS), Presbyterian Healthcare Services (PHS), Albuquerque, NM, USA-
bAdult Internal Medicine Services (AIMS), Presbyterian Healthcare Services (PHS), Albuquerque, NM, USA
cDepartment of Hematology/Oncology, Presbyterian Healthcare Services (PHS), Albuquerque, NM, USA
dDepartment of Hematology/Medical Oncology, UPMC Hillman Cancer Center, Indiana, PA, USA
eCorresponding Author: Muralidhar Idamakanti, Adult Inpatient Medical Services (AIMS), Presbyterian Healthcare Services (PHS), Albuquerque, NM, USA
Manuscript submitted April 29, 2025, accepted May 29, 2025, published online June 16, 2025
Short title: HER2-Negative Breast Cancer With Trastuzumab
doi: https://doi.org/10.14740/jmc5136
| Abstract | ▴Top |
The human epidermal growth factor receptor 2 (HER2)/erythroblastic oncogene B2 (ERBB2) is a tyrosine kinase receptor protein that plays an important role in the pathogenesis and aggressive nature of the tumors. It is well studied in various cancers, including breast, gastric, esophageal, ovarian, lung, and endometrial cancers. It is a well-known negative prognostic indicator in breast cancer associated with decreased disease-free survival and overall survival. Breast cancer treatment has been revolutionized with the invention of targeted monoclonal antibody therapies against the HER2 receptor, particularly trastuzumab and its antibody-drug conjugates (ADCs). HER2-targeted therapies have proven to improve progression-free survival and overall survival when added to chemotherapy in adjuvant, neoadjuvant, and metastatic settings in patients who are HER2-positive. ADCs approved for breast cancer include trastuzumab emtansine (Kadcyla, T-DM1) and trastuzumab deruxtecan (Enhertu, T-DXd). With enthusiasm and reported benefit, particularly in metastatic disease, HER2-targeted therapies are now widely used in breast cancer patients classified as HER2-negative based on binary classification but categorized as “HER2-low” with some degree of HER2 expression. HER2-targeted therapies are not approved for patients who have HER2 (ERBB2) mutation and have no HER2 expression of any degree (immunohistochemistry (IHC) 0+). We could not find any such reported cases, research studies, or clinical trials of HER2-targeted therapies being used in patients with HER2 mutation in our extensive search of the literature. We present a rare practice-changing case of a patient with triple negative metastatic breast cancer (estrogen receptor (ER), progesterone receptor (PR), and HER2 negative, HER2 0+ on IHC) and positive HER2 mutation, who achieved a disease-free survival of more than 2.5 years with trastuzumab deruxtecan monotherapy. This case makes a compelling argument for considering HER2-targeted therapies, mainly ADCs, in HER2-mutant breast cancer patients either as a monotherapy or in combination with other therapies, particularly in metastatic disease. This case report also indicates that further research and clinical trials looking into the efficacy and safety profile of anti-HER2 treatments in HER2-mutant breast cancers are warranted.
Keywords: Human epidermal growth factor receptor 2; HER2-targeted therapies; HER2 mutation; Pertuzumab; Antibody-drug conjugates; Trastuzumab deruxtecan; Trastuzumab emtansine; Immunohistochemistry; Metastatic breast cancer; HER2-low breast cancer; Non-small-cell lung cancer; Bispecific antibodies
| Introduction | ▴Top |
The human epidermal growth factor receptor 2 (HER2)/erythroblastic oncogene B (ERBB2) is a 185-kDa transmembrane receptor glycoprotein with tyrosine kinase activity encoded by the HER2 oncogene located on the long arm of chromosome 17 [1-3]. The overexpression of HER2 will stimulate intrinsic kinase activity, which activates cellular signal cascades that eventually promote cellular multiplication, survival, and angiogenesis, which are important in the tumor pathogenesis [1, 3, 4]. It is also responsible for the aggressive nature of cancer by promoting the tumor invasion and metastasis [1, 4, 5]. The scope of management in breast cancer is widened with the invention of novel HER2-targeted therapies. Even though HER2 is expressed in breast, gastric, esophageal, lung, colon, ovarian, endometrial, and head and neck cancers, promising outcomes with HER2-targeted therapies are only seen in HER2-positive and HER2-low breast cancers, advanced HER2-positive gastroesophageal cancers, and HER2-mutant non-small-cell lung cancers (NSCLCs) [2, 6]. Trastuzumab and other HER2-targeted therapies (antibody-drug conjugates (ADCs)) in breast cancers with HER2 mutation and null HER2 expression are not well studied in the literature so far. By the time of this report, we could not find any such reported cases, research studies, or clinical trials in the literature. Here we report a case of a triple negative (HER2 0+ on immunohistochemistry (IHC)) metastatic breast cancer patient with HER2 mutation who so far achieved 3 years of invasive disease-free survival and relapse-free survival. We also reviewed the timeline of HER2-targeted therapies and the landmark studies that led to their approval and widespread use in breast cancer through an extensive literature review.
| Case Report | ▴Top |
Our patient is a 71-year-old postmenopausal, multiparous female with a medical history of type 2 diabetes mellitus, diabetic neuropathy, hyperlipidemia, osteoarthritis, and osteoporosis who had a diagnostic mammogram in March 2018 for left breast nipple inversion for 4 months, which showed a 1.9 × 3 × 2 cm irregular mass in the left breast. Targeted ultrasound showed an 8.3 × 3.5 × 5.5 cm irregular echogenic mass with irregular margins at the 1 o'clock position, with 7 cm depth from the nipple in the left breast. She was diagnosed with grade 2 pleomorphic lobular carcinoma of the left breast by core needle biopsy in January 2018. Further analysis showed estrogen receptor (ER) 100%, progesterone receptor (PR) negative, HER2 1+ on IHC, and Ki-67 protein 81%. She received neoadjuvant chemotherapy with four cycles of docetaxel (147 mg/cycle) and cyclophosphamide (1,175 mg/cycle) within the next 3 months. The patient then had a wide local excision of two left breast masses and axillary lymph node biopsy with results still positive for pleomorphic lobular carcinoma with positive 6 of 11 lymph nodes, graded as stage III (T3N2a) in May 2018. This was followed by a simple mastectomy with biopsy showing benign breast tissue in June 2018 and 30 sessions of definitive adjuvant radiotherapy (XRT) between August and September 2018, where she received a total of 50 Gy in 25 fractions to left chest wall and draining lymphatics followed by a 10 Gy boost in 5 fractions to the mastectomy scar for a total dose of 60 Gy.
After this, the patient received 2 months of letrozole 2.5 mg per os (PO) daily between September and November 2018 (discontinued secondary to myalgias, pre-syncope, dizziness, and right-hand swelling; improved after drug cessation), 10 months of anastrozole 1 mg PO daily between December 2018 and October 2019 (discontinued due to worsening musculoskeletal symptoms), and approximately 16 months of exemestane 25 mg PO daily between November 2019 and February 2021. In January 2021, the patient had a magnetic resonance imaging (MRI) scan of the brain due to unsteady gait and frequent falls, which showed a large 4.8 × 4.2 × 4 cm cerebellar mass with surrounding edema and underwent craniectomy and excisional biopsy (Fig. 1). Histopathology showed metastatic breast carcinoma, grading her tumor as stage IV. Pathology of the brain metastatic lesion was reported as triple negative (ER negative, PR negative, HER2 0+ on IHC). Next-generation sequencing came positive for BRCA2 and HER2 L755S mutations, probably from genomic loss of heterozygosity (LOH). Following this, the patient received five sessions of stereotactic radiation therapy (SRT) for a total of 25 Gy to brain metastatic lesions in February 2021.
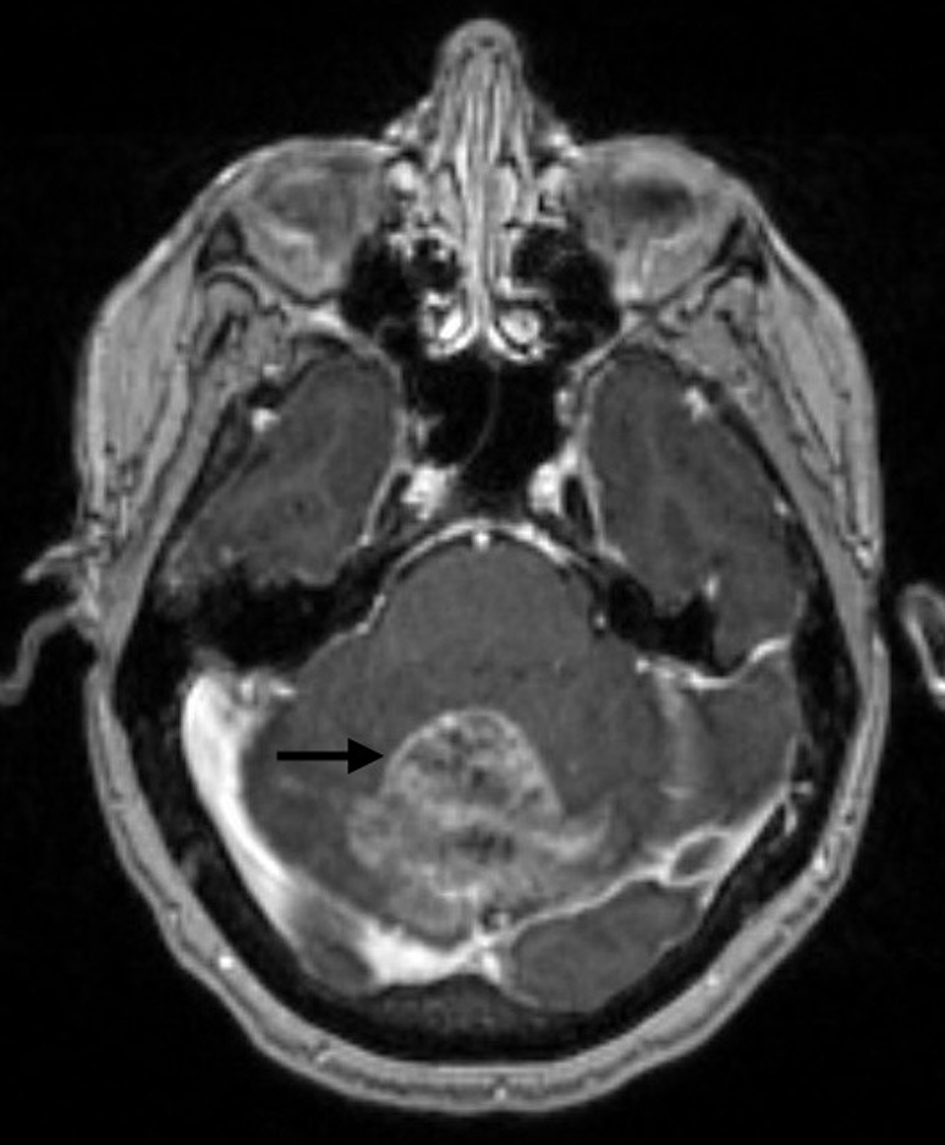 Click for large image | Figure 1. Brain MRI with and without contrast showing a 4.8 × 4.2 × 4 cm well-circumscribed enhancing mass (arrow) in the superior aspect of the left and right cerebellar hemispheres. MRI: magnetic resonance imaging. |
After this, the patient received 1 year of treatment with olaparib 300 mg twice daily (an inhibitor of poly ADP ribose polymerase (PARP) involved in DNA repair), mainly to target BRCA2 mutation [7]. A follow-up MRI scan in February 2022 showed progression of residual metastatic disease measuring 34 × 24 mm in the midline superior cerebellar regions (Fig. 2). Olaparib was discontinued in March 2022 due to the progression of brain metastasis. Radiation oncology was consulted, and the patient was not deemed a candidate for repeat SRT as the growth recurred in the exact same site, and whole-brain radiation therapy (WBRT) was deemed unnecessary as the expected adverse effects outweighed the benefits. The patient was hence started on a combination therapy with tucatinib (300 mg oral twice daily), capecitabine (1,000 mg/m2 oral twice daily with 2 weeks on and 1 week off), and trastuzumab infusion (608 mg every 3 weeks), which was discontinued after one cycle due to the decline in the performance status.
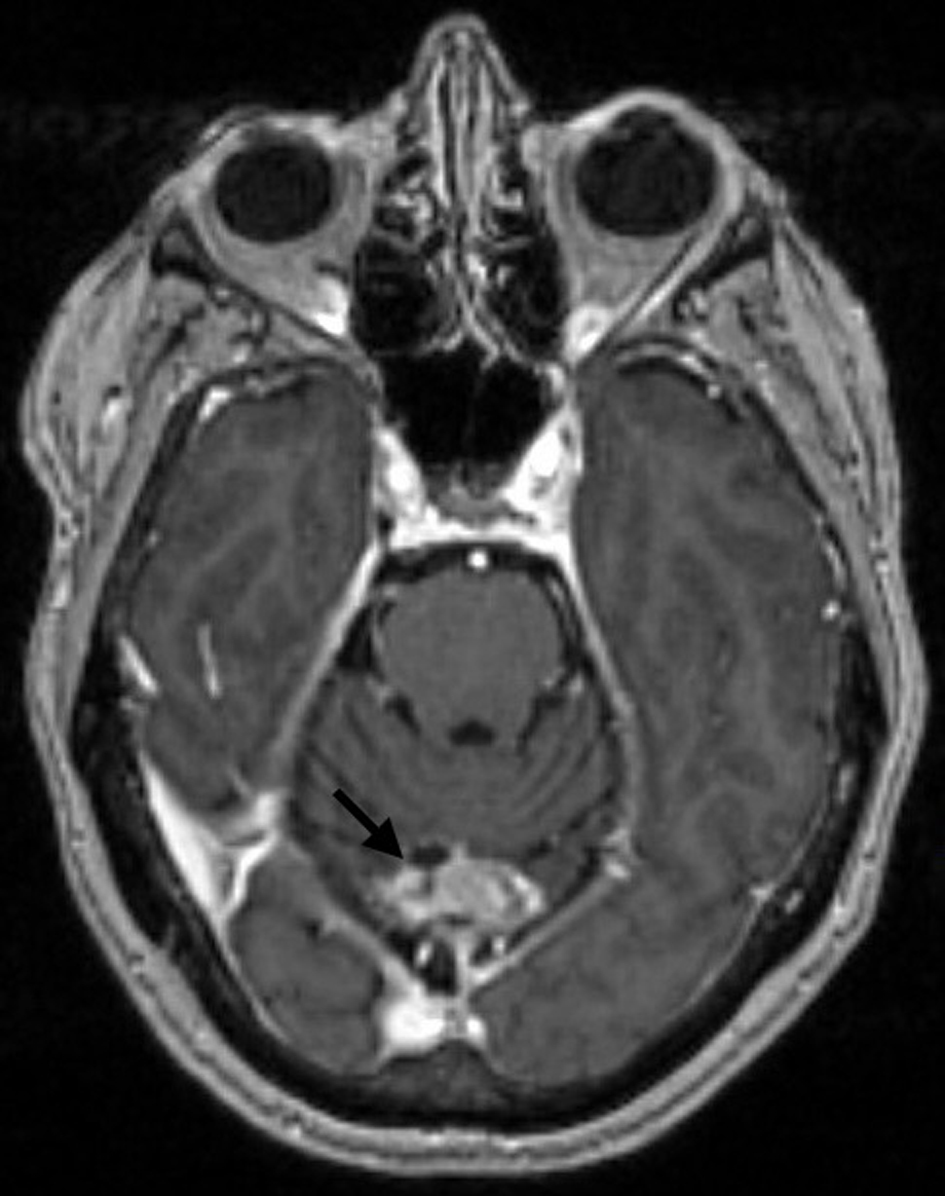 Click for large image | Figure 2. Brain MRI with and without contrast showing midline occipital craniectomy changes and interval progression in size and conspicuity of residual metastatic disease, measuring 3.4 × 2.4 cm (arrow). MRI: magnetic resonance imaging. |
At that time, based on the encouraging results from the DESTINY-Lung01 trial, promising early reports from the DESTINY-Breast04 trial on HER2-low breast cancer, and the early data from the DESTINY-PanTumor01 study, we hypothesized that trastuzumab deruxtecan could be effective for our patient with HER2 mutation and HER2 0 on IHC. After discussing with peers who had expertise in the HER2 breast cancer field, the patient was started on a reduced-dose monotherapy with trastuzumab deruxtecan 374.8 mg every 3 weeks in April 2022. Around the same time, the patient had a lung biopsy for a concerning lesion in the right upper lobe in May 2022, and the pathology showed benign lung with patchy acute inflammatory changes. Follow-up MRI scans of the brain in July 2022 and March 2023 showed improvement in the cerebellar metastatic findings (Fig. 3a, b). The dose of trastuzumab deruxtecan was decreased by one level due to fatigue after a year of treatment in April 2023. A pulmonologist was consulted for mild dyspnea and sleep hypoxia, and the patient had a high-resolution computed tomography (HRCT) scan of the chest in March 2024, which reported negative interstitial lung disease (ILD).
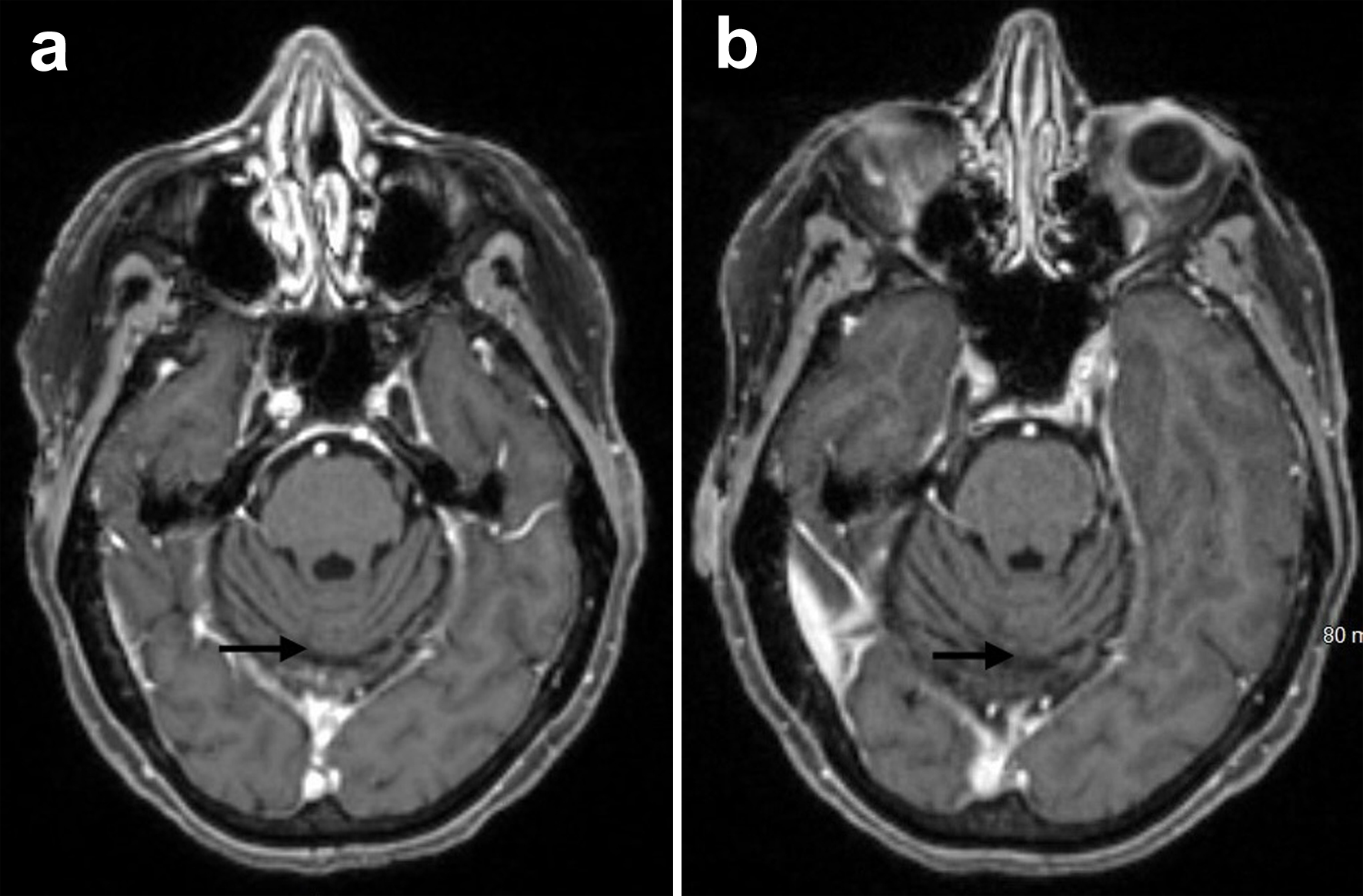 Click for large image | Figure 3. Brain MRI with and without contrast showing stable midline occipital postsurgical changes with interval resolution of recurrent parenchymal metastatic disease (arrows) from July 2022 (a) and March 2023 (b). MRI: magnetic resonance imaging. |
Serial brain MRIs reported stable findings, including the most recent one in December 2024 (Fig. 4a, b). Follow-up positron emission tomography (PET)-CT scans, including the most recent in January 2025, also showed no evidence of tumor recurrence or metastatic disease and stable right lung upper lobe inflammatory findings. Periodical multigated acquisition (MUGA) scan showed good heart function with an ejection fraction of 65-69%. The patient so far has achieved 3 years of invasive disease-free survival and relapse-free survival, and has been tolerating trastuzumab deruxtecan very well with good physical performance and without significant adverse events that would warrant the discontinuation of therapy.
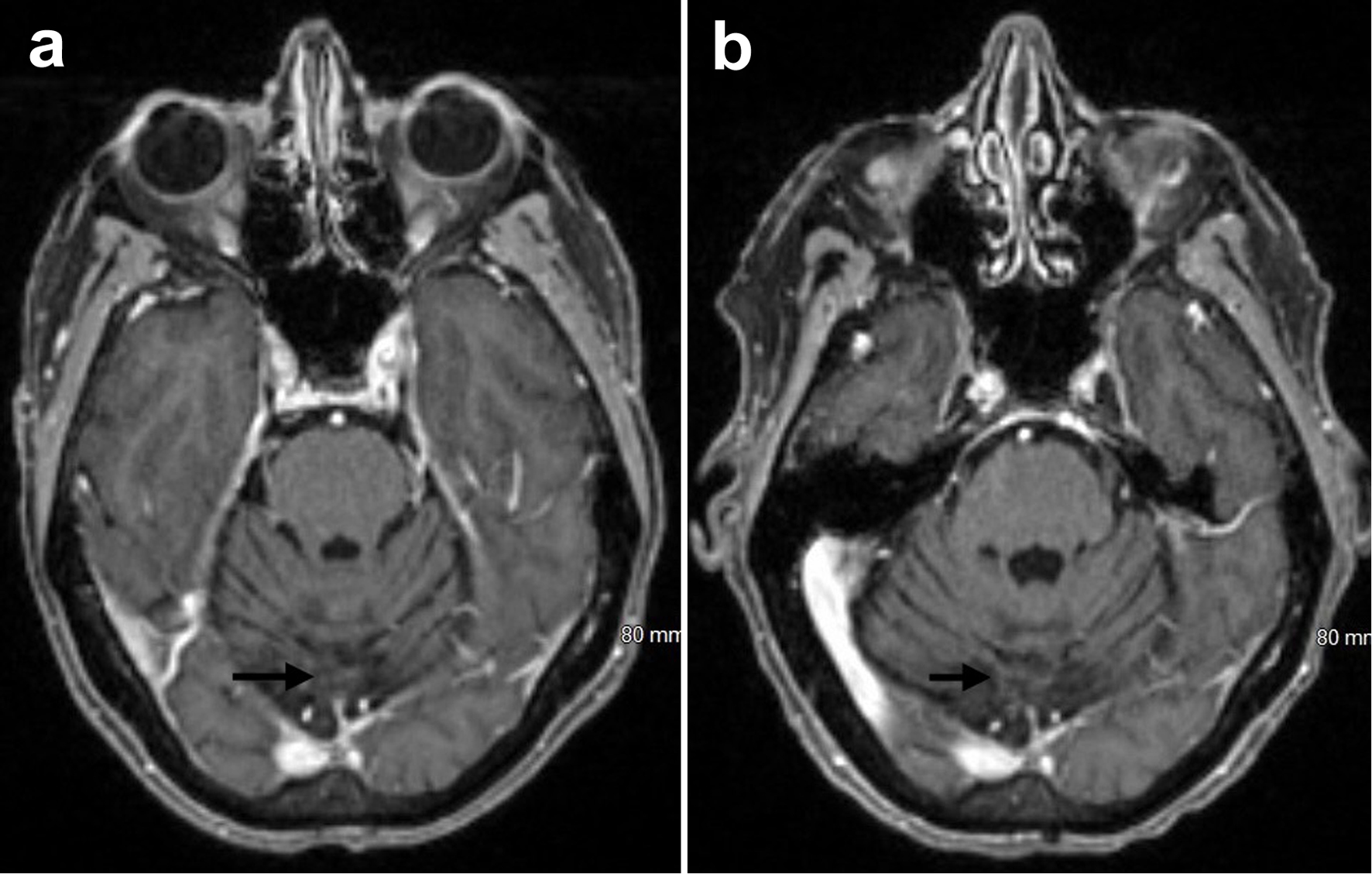 Click for large image | Figure 4. Brain MRI with and without contrast showing remote midline occipital postoperative changes with hemosiderin deposition and no evidence of parenchymal metastatic disease (arrows) from March 2024 (a) and December 2024 (b). MRI: magnetic resonance imaging. |
| Discussion | ▴Top |
The HER2/ERBB2 is a 185-kDa transmembrane receptor protein with tyrosine kinase activity encoded by the HER2 oncogene located on the long arm of chromosome 17 [1-3, 8]. The overexpression of HER2 will stimulate intrinsic kinase activity, which activates cellular signal cascades that eventually promote cellular multiplication, survival, and angiogenesis, which are important in the tumor pathogenesis [1-4]. It is also responsible for the excessive tumor growth and aggressive nature of cancer by promoting the tumor invasion and metastasis [1-3, 8, 9]. HER2 is a well-known negative prognostic indicator in breast cancer since its discovery in the 1980s, and its overexpression is known to cause shorter disease-free intervals and decreased overall survival [1, 3, 8, 9].
HER2 is expressed in 20-30% of breast cancers, making it a common target in breast cancer management [2, 8, 9]. These facts were well demonstrated in a study by Slamon and colleagues in 1987, which paved the way for research on HER2-targeted therapies. HER2 expression is routinely assessed using a combination of IHC (evaluates HER2 protein levels) and fluorescent in situ hybridization (FISH, evaluates HER2 gene status) [1, 2, 10]. HER2-positive or overexpression is described as an IHC score of 3+ or an IHC score of 2+ and gene amplification by FISH [2, 4, 10]. HER2-negative is described as IHC score of 0/1+ with or without gene amplification and IHC 2+ without gene amplification [2, 4, 10].
Breast cancer treatment has been revolutionized by the invention of targeted monoclonal antibody therapies against the HER2 receptor protein, particularly trastuzumab and its ADCs and pertuzumab, which positively modify the disease progression [1, 4, 5]. Trastuzumab targets the extracellular ligand-binding domain, and pertuzumab binds to the extracellular dimerization domain (subdomain II) of HER2 receptor; both in turn alter its normal tyrosine kinase pathway, which plays a main role in the pathogenesis of the disease [4, 10, 11]. ADCs approved for breast cancer include trastuzumab emtansine and trastuzumab deruxtecan. In HER2-positive breast cancer cases, HER2-targeted therapies decrease disease progression and promote disease-free survival and overall survival when added to chemotherapy in adjuvant, neoadjuvant, and metastatic settings. Below, in a chronological order, we briefly reviewed the timeline of HER2-targeted therapies and the landmark studies that led to their approval and widespread use.
Slamon et al in their study in 1987 demonstrated that HER2 was expressed in around 30% of breast cancer cases, and its expression is associated with tumor growth and poor prognosis [8]. In the coming years, Hudziak et al developed an HER2-targeted monoclonal antibody (anti-p185HER2) and showed that HER2-blocking antibodies may not only potentiate the effects of other breast cancer therapies but also are effective on their own [11]. The anti-p185HER2 antibody, which would later develop into trastuzumab, was humanized and used in phase I clinical trials in 1992 [12]. Trastuzumab was first approved by the FDA in 1998 to treat HER2+ metastatic breast cancer based on a groundbreaking phase III trial by Slamon et al. This study demonstrated that trastuzumab combined with chemotherapy significantly improved outcomes compared to chemotherapy alone, with results showing longer median time to progression, improved overall survival, and a comparative 20% lower risk of death [13]. Use of trastuzumab in HER2-overexpressing metastatic breast cancer was further supported by a multinational study reported by Shak in 1999 [14]. Baselga et al in the same year reported that the combination of paclitaxel and recombinant humanized anti-HER2 antibody (rhuMAb HER2) showed the largest rates of cancer growth inhibition and complete tumor regression when compared with the lone use of either one of them [15].
Seidman et al in their study reviewing cardiac dysfunction (CD) in trastuzumab trials reported in 2002 that trastuzumab is associated with increased risk of CD, particularly when used along with anthracyclines but its use in metastatic breast cancer is justified given the improvement in overall survival (more benefits compared to risks) and that CD could be managed clinically [16]. The HERA trial, published in 2005, reported that using trastuzumab for 1 year after standard chemotherapy significantly lowered the risk of recurrence and showed promising improvement in disease-free survival [17]. With the help of results from multiple studies, the FDA approved trastuzumab to treat early HER2-positive, node-positive breast cancer as adjuvant therapy after surgical excision with chemotherapy in 2006 [18].
In 2008, the ADC, trastuzumab emtansine, was developed and studied by Lewis Phillips et al. Results showed that trastuzumab emtansine was effective in trastuzumab-sensitive and trastuzumab-insensitive HER2-overexpressing breast cancer cases with a comparatively good pharmacokinetic and safety profile [19]. In a phase III clinical trial by the EMILIA study group in 2013, researchers reported that the use of trastuzumab emtansine in patients with metastatic breast cancer who were earlier treated with any HER2-targeted therapy showed an amazing 45% lower risk of disease progression or death compared with patients treated with lapatinib plus chemotherapy. The data from this study led to the FDA approval of trastuzumab emtansine for these types of patients in 2013 [20].
Key success of trastuzumab encouraged the researchers to develop another HER2-targeted therapy, pertuzumab, with a different mechanism of action as mentioned above. In a phase III study by the CLEOPATRA study group, researchers in 2012 reported that patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel showed a median progression-free survival (PFS) of 18.5 months compared to 12.4 months in similar patients treated with trastuzumab and docetaxel alone [21]. The TRYPHAENA study evaluated the safety and efficacy of pertuzumab and trastuzumab combined with chemotherapy as a neoadjuvant treatment for HER2-positive breast cancer, with the primary focus being cardiac safety. Results showed a low incidence of symptomatic left ventricular dysfunction (LVSD) and manageable decreases in ejection fraction [22]. Pathological complete response (pCR) rates were high, supporting the regimen’s effectiveness in this setting without major new safety concerns during the follow-ups [22, 23]. These results played a pivotal role in the FDA approval of the combination therapy of trastuzumab, pertuzumab, and docetaxel in 2012 for metastatic breast cancer patients with HER2 overexpression.
The KRISTINE study reported that the trastuzumab emtansine plus pertuzumab regimen was less effective than the standard trastuzumab, pertuzumab, and chemotherapy regimen for HER2-positive early breast cancer. The study found that patients receiving trastuzumab emtansine and pertuzumab had a higher risk of disease recurrence or progression than those receiving the standard treatment. However, trastuzumab emtansine plus pertuzumab was associated with fewer side effects than chemotherapy. While this combination was promising in terms of toxicity, the lower efficacy made the standard chemotherapy-based treatment the preferred option in early HER2-positive breast cancer settings [24, 25].
The adjuvant paclitaxel and trastuzumab (APT) trial demonstrated that the combination of paclitaxel and trastuzumab is an effective and safe adjuvant treatment for patients with stage I, node-negative, and HER2-positive breast cancer [26]. The regimen resulted in an impressive 5-year disease-free survival rate of 97%, a 10-year invasive disease-free survival rate of 91.3%, and a 10-year breast cancer-specific survival rate of 98.8% with a low risk of severe cardiac or other significant toxicities. The results supported using a less intensive adjuvant therapy for patients with smaller, node-negative tumors, emphasizing the potential to tailor treatments to reduce overtreatment. The trial also highlighted the potential of genomic tools, such as HER2DX, to refine prognostic assessments and guide more personalized treatment strategies, especially for selecting patients who need intensive versus less aggressive therapy [26, 27].
The KATHERINE trial evaluated the efficacy of trastuzumab emtansine compared to trastuzumab as adjuvant therapy in patients with HER2-positive early breast cancer who had residual invasive disease following neoadjuvant therapy. In 2018, the trial established trastuzumab emtansine as the preferred treatment over trastuzumab for patients with residual HER2-positive invasive disease after neoadjuvant therapy, offering superior efficacy in preventing recurrence and improving survival outcomes with an acceptable safety profile [28]. Trastuzumab emtansine significantly reduced the risk of invasive disease recurrence compared to trastuzumab. Breast cancer-specific deaths were also lower in the trastuzumab emtansine arm (9.5%) versus the trastuzumab arm (15%), but trastuzumab emtansine was associated with more adverse events, such as thrombocytopenia and peripheral neuropathy, though most were manageable and reversible [28].
With these promising results, researchers started looking at HER2-targeted therapies in patients with low HER2 expression, and with this enthusiasm, a different category of “HER2-low” breast cancers has been proposed and studied in the literature, described as cancers with HER2 1+/2+ on IHC and no gene amplification with FISH [1, 4, 5]. The development of novel HER2-targeted therapies, ADCs, particularly trastuzumab deruxtecan, has further increased the interest in this category of “HER2-low” breast cancers, which almost constitute 50% of all the breast cancers [29].
DESTINY-Breast01 trial showed that trastuzumab deruxtecan had durable antitumor activity with improved PFS in patients previously treated with HER2-targeted therapies, including trastuzumab emtansine. The data from this study led to the accelerated approval of trastuzumab deruxtecan for resistant advanced breast cancers that were previously treated with at least two HER2-targeted therapies in 2019 [30]. Full approval by the FDA came through in 2022 via the DESTINY-Breast03 trial in metastatic breast cancer, where authors reported that the disease progression and risk of death were lower in patients who received trastuzumab deruxtecan than those who received trastuzumab emtansine [31]. DESTINY-Breast04 trial, in advanced HER2-low breast cancers, demonstrated benefit with results showing median PFS of 9.9 months (95% CI) in the trastuzumab deruxtecan group compared to 5.1 months in the physician’s choice chemotherapy group [32]. Results from the DESTINY-Breast04 trial led to the FDA approval of trastuzumab deruxtecan for patients with HER2-low breast cancers in August 2022. Zimmerman et al in their recent study reported in 2024 concluded that ADCs in HER2-low breast cancers have notable improvement in patient outcomes [33]. With all these studies and advancements, HER2-targeted therapies are now widely used in HER2-low breast cancer patients.
Metastatic central nervous system (CNS) disease in breast cancer is a separate entity itself, given the complexity of treatment decisions with surgery, radiation, and systemic treatment. Management of brain metastases in breast cancer is guided by factors such as tumor subtype (HER2-positive, triple-negative, or hormone receptor-positive), number and location of lesions, performance status, and systemic disease control. Multidisciplinary evaluation is essential to tailor therapy based on individual clinical and molecular factors. For patients with limited brain metastases (typically ≤ 4 lesions), SRT is preferred due to its precision and preservation of cognitive function. WBRT may be used in cases of multiple metastases or leptomeningeal disease, but it is associated with significant cognitive decline. Surgical resection is considered for symptomatic or large solitary lesions [34]. Systemic therapy is increasingly important, especially in HER2-positive disease, where agents like HER2-targeted therapies (ADCs) [35-38], PARP inhibitors (olaparib) [7], and tyrosine kinase inhibitors (like tucatinib [39], pyrotinib [40]) have demonstrated intracranial activity and improved survival (Table 1). For triple-negative and HR-positive subtypes, systemic options are more limited, but ongoing trials are expanding treatment choices.
 Click to view | Table 1. Summary of Important Systemic Therapies Used in CNS Metastatic Breast Cancer |
The DEBBRAH trial, reported through 2023 - early 2025, demonstrated that trastuzumab deruxtecan (T-DXd) has meaningful intracranial activity in patients with HER2-positive and HER2-low metastatic breast cancer across cohorts with active brain metastases, stable CNS disease, or leptomeningeal disease. T-DXd showed intracranial objective response rates (ORRs) ranging from 42% to 58% and disease-control rates (DCRs) exceeding 80% in most groups. Notably, responses were seen even in HER2-low tumors, a population with previously limited options. Additionally, patients with leptomeningeal metastases, a subgroup with particularly poor prognosis, experienced clinical benefit and prolonged stability. These findings confirm the broad CNS efficacy of T-DXd and support its use in both HER2-positive and HER2-low patients with CNS involvement, but further larger studies are needed to validate these results [35-37]. The TUXEDO-1 trial, a recent phase II study evaluating trastuzumab deruxtecan (T-DXd) in patients with HER2-positive breast cancer and active brain metastases, demonstrated a high intracranial response rate of 73.3% and PFS of 21 months, supporting the use of ADCs as systemic therapy for active brain metastases [38] (Table 1).
HER2-targeted therapies are not approved for breast cancer patients who have HER2 (ERBB2) mutation and have no degree of HER2 expression (IHC 0+). The literature review showed only a few studies or trials where HER2-targeted therapies were tried in other (non-breast) cancers with HER2 mutations. Trastuzumab deruxtecan is approved by the FDA and the European Medicines Agency for HER2-mutant NSCLC because of promising results from the DESTINY-Lung01 trial [6]. In the DESTINY-Lung01 trial, reported in September 2021, investigators proved that trastuzumab deruxtecan had shown sustainable anticancer activity in patients with previously treated HER-2 mutant NSCLC [6]. In the DESTINY-PanTumor01 study, reported in May 2024, trastuzumab deruxtecan showed decent antitumor activity with a sustainable response in previously treated patients across various solid tumors harboring HER2 mutation, and further investigation is needed before using trastuzumab deruxtecan in the pan-tumor setting [41].
In a phase II trial studying the efficacy of the drug poziotinib, a pan-HER inhibitor that irreversibly blocks the signaling pathways of epidermal growth factor receptor (EGFR), HER2, and HER4, in patients with HER2 exon-20 mutant NSCLC, Elamin et al in 2021 reported that poziotinib showed promising antitumor activity [42]. Similar results were noted by Wang et al in their pooled analysis of randomized clinical trials in 2022 [43]. Patients are actively being recruited for a phase II clinical trial in China to evaluate the efficacy and safety of disitamab vedotin (ADC) plus cadonilimab (immune checkpoint inhibitor) in second-line treatment of patients with HER2-mutant advanced or metastatic bile duct adenocarcinoma, last updated in April 2024 [44].
In our extensive search of the literature, we could not find any reported cases, research studies, or clinical trials where HER2-targeted therapies are used in patients with HER2 mutation and negative HER2 expression. We hereby present a rare case report of a patient with triple-negative metastatic breast cancer and positive HER2 mutation, who so far has achieved disease-free survival of 3 years with trastuzumab deruxtecan monotherapy. Our patient was categorized as triple negative (ER, PR, and HER2-negative) with HER2 0+ on IHC and HER2 mutation-positive from the biopsy and next-generation sequencing results of the metastatic brain lesion. This patient was started on trastuzumab deruxtecan monotherapy in April 2022 after failed and intolerant treatments for her stage IV disease with recurrent metastatic brain lesions as detailed above in the history, and so far, has been disease-free for 3 years. Serial brain MRI scans reported improved brain findings, and follow-up PET-CT scans showed no evidence of tumor recurrence or metastatic disease, and stable chronic inflammatory findings of the right lung. Periodical MUGA scan showed good heart function with ejection fraction of 65-69%. Patient is also tolerating trastuzumab deruxtecan well with good physical performance and without significant adverse events that warrant discontinuation of therapy.
This case could be used as an example to argue or consider the use of HER2-targeted therapies, mainly ADCs, in HER2-mutant breast cancer patients (particularly with metastatic disease) who have no HER2 expression and have responded poorly or have been intolerant to conventional therapies. We acknowledge the limitation of applying the results from this single case to a broader clinical practice, but we want to make a valiant point that this is an interesting arena to explore and stress the need for further clinical research and larger trials before a recommendation or change of guidelines could be made. Bispecific antibodies combining both trastuzumab and pertuzumab or targeting both HER2 and HER3 receptors or an antibody with two drug conjugates are some of the future perspectives in HER-targeted therapies for breast cancer, particularly for HER2-mutant breast cancer patients with null HER2 expression.
Conclusion
Targeted monoclonal antibody therapies against HER2, trastuzumab and its ADCs, have revolutionized breast cancer treatment. HER2-targeted therapies have proven to decrease disease progression and promote disease-free survival and overall survival when added to chemotherapy in adjuvant, neoadjuvant, and metastatic settings in patients who are HER2-positive. ADCs, particularly trastuzumab deruxtecan, are now widely used even in breast cancer patients classified as “HER2-low”. However, HER2-targeted therapies are not approved for patients with an HER2 mutation and no degree of HER2 expression (IHC 0+). Our case report on this patient, with triple-negative metastatic breast cancer and positive HER2 mutation, who achieved invasive disease-free survival and relapse-free survival of 3 years with trastuzumab deruxtecan monotherapy, makes a compelling argument for considering HER2-targeted therapies, mainly ADCs, in HER2-mutant breast cancer patients either as a monotherapy or combination with other therapies, particularly in patients who have responded poorly or have been intolerant to the conventional therapies. This practice-changing observation opens an interesting area of study on HER2-targeted therapies in HER2-mutant breast cancers without HER2 expression. Further research and clinical trials looking into the efficacy and safety profile of anti-HER2 treatments in HER2-mutant and triple-negative breast cancers are warranted before any treatment guidelines can be proposed. Bispecific antibodies also form the future perspectives in HER-targeted therapies for resistant metastatic breast cancer.
Learning points
Learning points from our report are to understand the role of HER2 in the pathogenesis of breast cancer and its implications in management, and historical review of HER2-targeted therapies, trastuzumab and its ADCs (trastuzumab emtansine and trastuzumab deruxtecan), in the treatment of breast cancer, and the landmark studies that led to their approval. In the process, we reviewed the path that led to the use of trastuzumab deruxtecan in HER2-low metastatic breast cancer, particularly CNS disease, and implicated its use in triple-negative metastatic breast cancer with HER2 mutation, an interesting area for future research and clinical trials (Fig. 5).
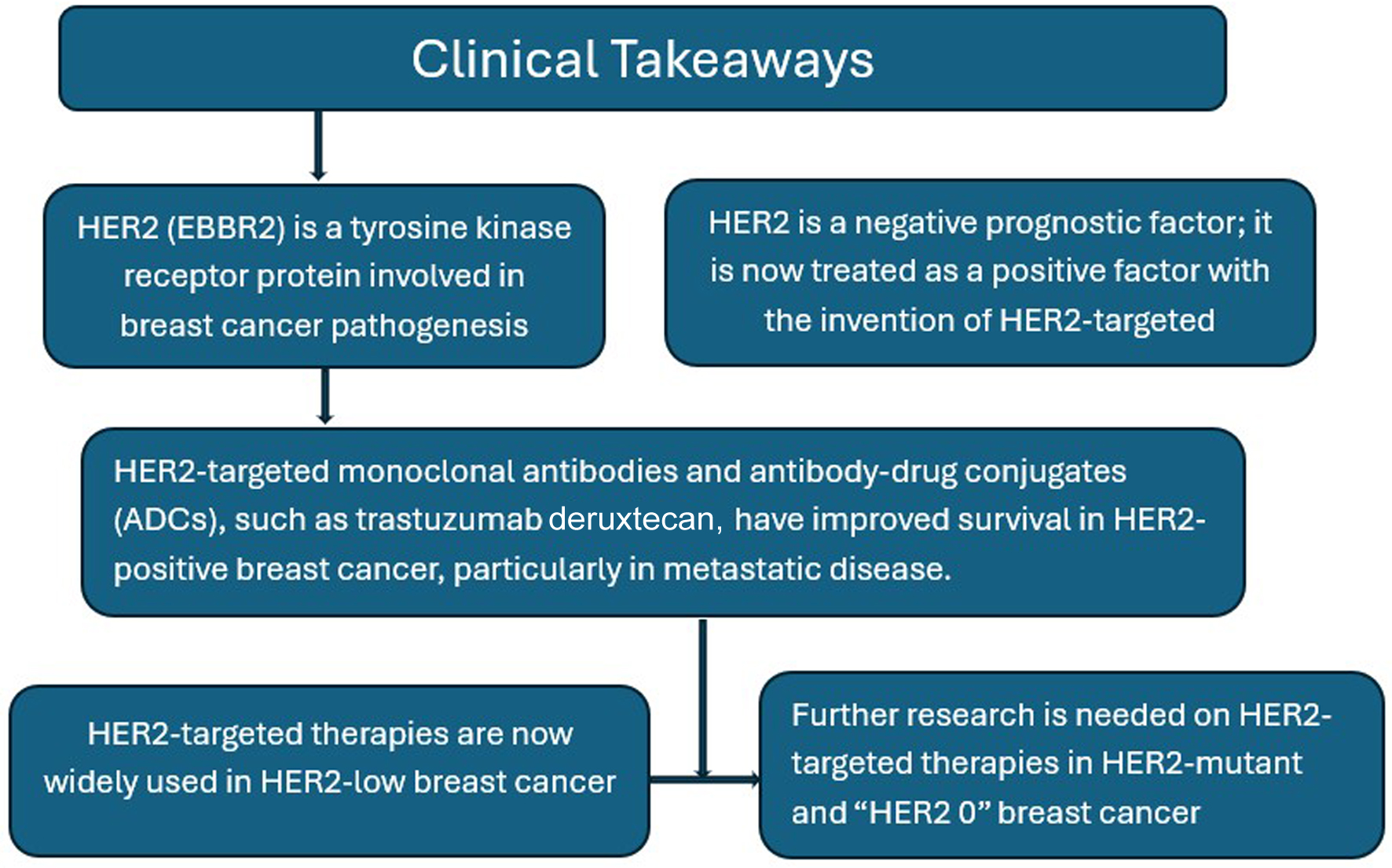 Click for large image | Figure 5. Graphical representation of clinical takeaways. |
Acknowledgments
None to declare.
Financial Disclosure
The authors declare that they do not have a financial relationship with any commercial entity that is interested in the subject of this manuscript.
Conflict of Interest
The authors declare that they do not have any conflict of interest.
Informed Consent
This manuscript does not use patient identifiers or pictures of the patient. The patient’s treating oncologist, who is a co-author in this report, obtained verbal consent from the patient.
Author Contributions
All the authors participated actively in various sections of this manuscript prior to submission. Dr. Idamakanti (primary author) started the case report, designed the article, and actively wrote and edited multiple sections of the manuscript, including abstract, case report, discussion, and conclusions, along with a literature review pertinent to the above sections. Dr. Kumar (co-author, corresponding oncologist) identified the rarity of the case and participated in the manuscript editing, writing the case report, and literature review. Dr. Bijjam (co-author) participated in manuscript editing, correcting grammar, and literature review, and helped with obtaining MRI images. Dr. Mukkamalla (co-author) participated in article design, manuscript editing, and literature review.
Data Availability
The authors declare that data supporting the findings of this case report are available within the article.
Abbreviations
ADC: antibody-drug conjugate; CD: cardiac dysfunction; CNS: central nervous system; ER: estrogen receptor; ERBB2: erythroblastic oncogene B2; FDA: Food and Drug Administration; FISH: fluorescent in situ hybridization; HER2: human epidermal growth factor receptor 2; HER2DX: genomic test that uses a supervised learning algorithm to predict likelihood of pathological complete response (pCR); HRCT: high-resolution computed tomography; IHC: immunohistochemistry; ILD: interstitial lung disease; LOH: loss of heterozygosity; LVSD: left ventricular systolic dysfunction; MRI: magnetic resonance imaging; MUGA: multigated acquisition; NSCLC: non-small-cell lung cancer; PARP: poly ADP ribose polymerase; PET: positron emission tomography; PR: progesterone receptor; SRT: stereotactic radiation therapy; WBRT: whole brain radiation therapy
| References | ▴Top |
- Zhang H, Katerji H, Turner BM, Hicks DG. HER2-low breast cancers. Am J Clin Pathol. 2022;157(3):328-336.
doi pubmed - Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int. 2014;2014:852748.
doi pubmed - Maadi H, Soheilifar MH, Choi WS, Moshtaghian A, Wang Z. Trastuzumab mechanism of action; 20 years of research to unravel a dilemma. Cancers (Basel). 2021;13(14):3540.
doi pubmed - Marchio C, Annaratone L, Marques A, Casorzo L, Berrino E, Sapino A. Evolving concepts in HER2 evaluation in breast cancer: heterogeneity, HER2-low carcinomas and beyond. Semin Cancer Biol. 2021;72:123-135.
doi pubmed - Vranic S, Beslija S, Gatalica Z. Targeting HER2 expression in cancer: new drugs and new indications. Bosn J Basic Med Sci. 2021;21(1):1-4.
doi pubmed - Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazieres J, Nagasaka M, et al. Trastuzumab Deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med. 2022;386(3):241-251.
doi pubmed - Cortesi L, Rugo HS, Jackisch C. An overview of PARP inhibitors for the treatment of breast cancer. Target Oncol. 2021;16(3):255-282.
doi pubmed - Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177-182.
doi pubmed - Aguilar Z, Akita RW, Finn RS, Ramos BL, Pegram MD, Kabbinavar FF, Pietras RJ, et al. Biologic effects of heregulin/neu differentiation factor on normal and malignant human breast and ovarian epithelial cells. Oncogene. 1999;18(44):6050-6062.
doi pubmed - Paik S, Kim C, Wolmark N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med. 2008;358(13):1409-1411.
doi pubmed - Hudziak RM, Lewis GD, Winget M, Fendly BM, Shepard HM, Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. 1989;9(3):1165-1172.
doi pubmed - Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, Rowland AM, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A. 1992;89(10):4285-4289.
doi pubmed - Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783-792.
doi pubmed - Shak S. Overview of the trastuzumab (Herceptin) anti-HER2 monoclonal antibody clinical program in HER2-overexpressing metastatic breast cancer. Herceptin Multinational Investigator Study Group. Semin Oncol. 1999;26(4 Suppl 12):71-77.
pubmed - Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 1998;58(13):2825-2831.
pubmed - Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, Murphy M, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20(5):1215-1221.
doi pubmed - Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659-1672.
doi pubmed - https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5250lbl.pdf.
- Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, Blattler WA, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68(22):9280-9290.
doi pubmed - Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783-1791.
doi pubmed - Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, Campone M, Ciruelos E, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14(6):461-471.
doi pubmed - Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, Tausch C, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24(9):2278-2284.
doi pubmed - Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Waldron-Lynch M, Eng-Wong J, et al. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: Evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur J Cancer. 2018;89:27-35.
doi pubmed - Hurvitz SA, Martin M, Jung KH, Huang CS, Harbeck N, Valero V, Stroyakovskiy D, et al. Neoadjuvant trastuzumab emtansine and pertuzumab in human epidermal growth factor receptor 2-positive breast cancer: three-year outcomes from the phase III KRISTINE study. J Clin Oncol. 2019;37(25):2206-2216.
doi pubmed - de Haas SL, Slamon DJ, Martin M, Press MF, Lewis GD, Lambertini C, Prat A, et al. Tumor biomarkers and efficacy in patients treated with trastuzumab emtansine + pertuzumab versus standard of care in HER2-positive early breast cancer: an open-label, phase III study (KRISTINE). Breast Cancer Res. 2023;25(1):2.
doi pubmed - Waks AG, Tolaney SM. The evolving understanding of small HER2-positive breast cancers: matching management to outcomes. Future Oncol. 2015;11(24):3261-3271.
doi pubmed - Tolaney SM, Tarantino P, Graham N, Tayob N, Pare L, Villacampa G, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer: final 10-year analysis of the open-label, single-arm, phase 2 APT trial. Lancet Oncol. 2023;24(3):273-285.
doi pubmed - von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, Wolmark N, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617-628.
doi pubmed - Nicolo E, Boscolo Bielo L, Curigliano G, Tarantino P. The HER2-low revolution in breast oncology: steps forward and emerging challenges. Ther Adv Med Oncol. 2023;15:17588359231152842.
doi pubmed - Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, Andre F, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610-621.
doi pubmed - Cortes J, Kim SB, Chung WP, Im SA, Park YH, Hegg R, Kim MH, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386(12):1143-1154.
doi pubmed - Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, Tsurutani J, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9-20.
doi pubmed - Zimmerman BS, Esteva FJ. Next-generation HER2-targeted antibody-drug conjugates in breast cancer. Cancers (Basel). 2024;16(4):800.
doi pubmed - Soffietti R, Abacioglu U, Baumert B, Combs SE, Kinhult S, Kros JM, Marosi C, et al. Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro Oncol. 2017;19(2):162-174.
doi pubmed - Perez-Garcia JM, Vaz Batista M, Cortez P, Ruiz-Borrego M, Cejalvo JM, de la Haba-Rodriguez J, Garrigos L, et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: The DEBBRAH trial. Neuro Oncol. 2023;25(1):157-166.
doi pubmed - Vaz Batista M, Perez-Garcia JM, Garrigos L, Garcia-Saenz JA, Cortez P, Racca F, Blanch S, et al. The DEBBRAH trial: Trastuzumab deruxtecan in HER2-positive and HER2-low breast cancer patients with leptomeningeal carcinomatosis. Med. 2025;6(1):100502.
doi pubmed - Vaz Batista M, Perez-Garcia JM, Cortez P, Garrigos L, Fernandez-Abad M, Gion M, Martinez-Bueno A, et al. Trastuzumab deruxtecan in patients with previously treated HER2-low advanced breast cancer and active brain metastases: the DEBBRAH trial. ESMO Open. 2024;9(9):103699.
doi pubmed - Bartsch R, Berghoff AS, Furtner J, Marhold M, Bergen ES, Roider-Schur S, Mair MJ, et al. Final outcome analysis from the phase II TUXEDO-1 trial of trastuzumab-deruxtecan in HER2-positive breast cancer patients with active brain metastases. Neuro Oncol. 2024;26(12):2305-2315.
doi pubmed - Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, Lin NU, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597-609.
doi pubmed - Yang Z, Meng J, Mei X, Mo M, Xiao Q, Han X, Zhang L, et al. Brain radiotherapy with pyrotinib and capecitabine in patients with ERBB2-positive advanced breast cancer and brain metastases: a nonrandomized phase 2 trial. JAMA Oncol. 2024;10(3):335-341.
doi pubmed - Li BT, Meric-Bernstam F, Bardia A, Naito Y, Siena S, Aftimos P, Anderson I, et al. Trastuzumab deruxtecan in patients with solid tumours harbouring specific activating HER2 mutations (DESTINY-PanTumor01): an international, phase 2 study. Lancet Oncol. 2024;25(6):707-719.
doi pubmed - Elamin YY, Robichaux JP, Carter BW, Altan M, Gibbons DL, Fossella FV, Lam VK, et al. Poziotinib for patients with HER2 exon 20 mutant non-small-cell lung cancer: results from a phase II trial. J Clin Oncol. 2022;40(7):702-709.
doi pubmed - Wang BC, Kuang BH, Liu XX, Lin GH. Poziotinib in non-small-cell lung cancer patients with HER2 exon 20 mutations: A pooled analysis of randomized clinical trials. Medicine (Baltimore). 2022;101(44):e31337.
doi pubmed - Disitamab Vedotin plus Cadonilimab in patients with HER2 mutant advanced or metastatic bile duct adenocarcinoma. https://clinicaltrials.gov/study/NCT06383533.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.