| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com |
Case Report
Volume 16, Number 4, April 2025, pages 153-157
Pulmonary Arterial Hypertension in a Patient With Metastatic Lung Cancer on Pembrolizumab: Whom to Blame?
Lakshmi Manogna Chintalacheruvua, d, Vamsi Krishna Chillurub, Narendra Babu Guttac
aDepartment of Hematology/Oncology, Southern Illinois Health Cancer Institute, Carterville, IL, USA
bDepartment of Nephrology, Southern Illinois Health, Carbondale, IL, USA
cDepartment of Interventional Radiology, Cape Radiology Group Inc, Cape Girardeau, MO, USA
dCorresponding Author: Lakshmi Manogna Chintalacheruvu, Department of Hematology/Oncology, Southern Illinois Health Cancer Institute, Carterville, IL 62918, USA
Manuscript submitted February 11, 2025, accepted April 18, 2025, published online April 26, 2025
Short title: PAH in Metastatic Lung Cancer on Pembrolizumab
doi: https://doi.org/10.14740/jmc5115
| Abstract | ▴Top |
Immune checkpoint inhibitors (ICIs) play a major role in current cancer treatments. They are associated with immune-mediated side effects due to immune dysregulation. ICI-mediated complications are more commonly known to affect thyroid gland, gastrointestinal system, skin, etc. Pulmonary arterial hypertension (PAH) due to ICIs is not very well described in the literature. Here, we report a case of severe PAH diagnosed in a patient with metastatic lung cancer on long-term pembrolizumab and literature review.
Keywords: Pulmonary hypertension; Immune checkpoint inhibitors; Pembrolizumab; Lung cancer; Pulmonary artery diameter; Aorta diameter
| Introduction | ▴Top |
Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment since its approval [1]. They predominantly target immunoregulatory molecules such as programmed cell death-1 (PD-1), program cell death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) [1]. Pembrolizumab is an ICI that targets the PD-1 pathway, thereby activating T cells to attack cancer cells [2]. This can cause immune dysregulation with increased inflammation and increased tissue damage, which can happen in any organ [1]. Common side effects associated with pembrolizumab include fatigue (19.4%), pruritus (10.7%), poor appetite (10.5%), and rash (9.7%) [3]. Pulmonary side effects such as pneumonitis (3.6%) and cough (2.4%) are rare and pulmonary arterial hypertension (PAH) is not highly reported in clinical trials [3]. Here, we report a case of severe PAH diagnosed in a patient with metastatic lung cancer on ICI pembrolizumab.
| Case Report | ▴Top |
A 71-year-old male with a past medical history of tobacco use, who quit more than 24 years ago with no other significant comorbidities, was referred to the clinic for newly diagnosed lung cancer. He initially noticed a lump in the right axilla, progressively increasing in size over the period of 2 months. Axillary ultrasound showed a 3.7 cm enlarged lymph node (LN) in the right axilla with effacement and hypervascularity. LN biopsy showed carcinoma with cytokeratin 7 (CK-7) and thyroid transcription factor-1 (TTF-1) positivity. P40 is focally positive, indicating findings consistent with lung adenocarcinoma, with a possibility of squamous component. Positron emission tomography (PET) scan showed multiple F-fluorodeoxyglucose (FDG) avid nodes in the right hilum, thoracic inlet, mediastinum, and right axilla concerning metastatic disease. Brain magnetic resonance imaging (MRI) did not show intracranial metastatic disease. Next-generation sequencing (NGS) showed high tumor mutational burden (TMB) with no other targetable mutations. Given the low disease burden and patient’s lack of interest in chemotherapy, the patient was started on single agent pembrolizumab with good treatment response. Seventeen months after initiating treatment, the patient presented to the clinic with worsening shortness of breath (SOB) which he noted a few months ago, was mild in the beginning, and has progressively gotten worse in the last few weeks. Most recent PET scan did not show any evidence of disease progression or signs of inflammation in lungs. An enlarged pulmonary artery diameter (PAD) measuring 35.96 mm with an aorta diameter (AoD) of 33.23 mm and PAD/AoD of 1.08 was shown in Figure 1, whereas PAD at the time of diagnosis was 31.67 mm, and AoD at the time of diagnosis was 33.92 mm, with a ratio of 0.93 as shown in Figure 2. Echocardiography (ECHO) was done, which showed normal left ventricular cavity size and ejection fraction of 55% with no regional wall motion abnormalities. Right ventricle (RV) cavity was markedly increased, with elevated right ventricular systolic pressure (RVSP) at 86 mm Hg with systolic pressure markedly elevated in pulmonary arteries consistent with severe PAH. No other significant abnormalities were found in aorta, tricuspid valve, mitral valve, and aortic valve, with mild regurgitation noted at pulmonary valve with no structural abnormalities. Later, he underwent right heart catheterization which showed predominant precapillary PAH with elevated pulmonary artery systolic pressure (PASP) at 83 mm Hg (normal range: 18 - 25 mm Hg) with mean value of 58 mm Hg, pulmonary vascular resistance (PVR) of 10.5 Wood Units (WU) (normal range: 0.25 - 1.6 WU), and left ventricular end diastolic pressure (LVEDP) of 20 mm Hg (normal range: 4 - 12 mm Hg). Left main had 50% stenosis with patent left anterior descending artery, left circumflex, and right coronary arteries. Ventilation perfusion scan was within normal limits. Labs ordered for connective tissue and autoimmune disease were also within normal limits including antibodies against antinuclear antibody (ANA), anti-neutrophil cytoplasmic antibodies (ANCA), Ku, Mi-2, OJ, PL-12, PL-7, signal recognition protein (SRP), U1 ribonucleoprotein (U1 RNP), U2 small nuclear ribonucleoprotein (U2 snRNP), Jo-1, cyclic citrullinated peptide (CCP), extractable nuclear antigen (ENA), fibrillarin, melanoma differentiation-associated 5 (MDA-5) P140, myeloperoxidase (MPO), NXP-2 P140, P155/140, polymyositis-scleroderma (PM-SCL), proteinase 3, and rheumatoid factor. He was evaluated by pulmonology. Given the overall clinical presentation with predominant group 1 disease, immunotherapy-induced PAH was diagnosed as a more likely diagnosis of exclusion and pembrolizumab was discontinued. He was later started on riociguat and macitentan after which exertional fatigue and dyspnea improved. Repeated ECHO showed normal RV size. Later, the patient switched to single agent pemetrexed and is clinically tolerating treatments well with no evidence of disease progression. His overall exercise tolerance has improved.
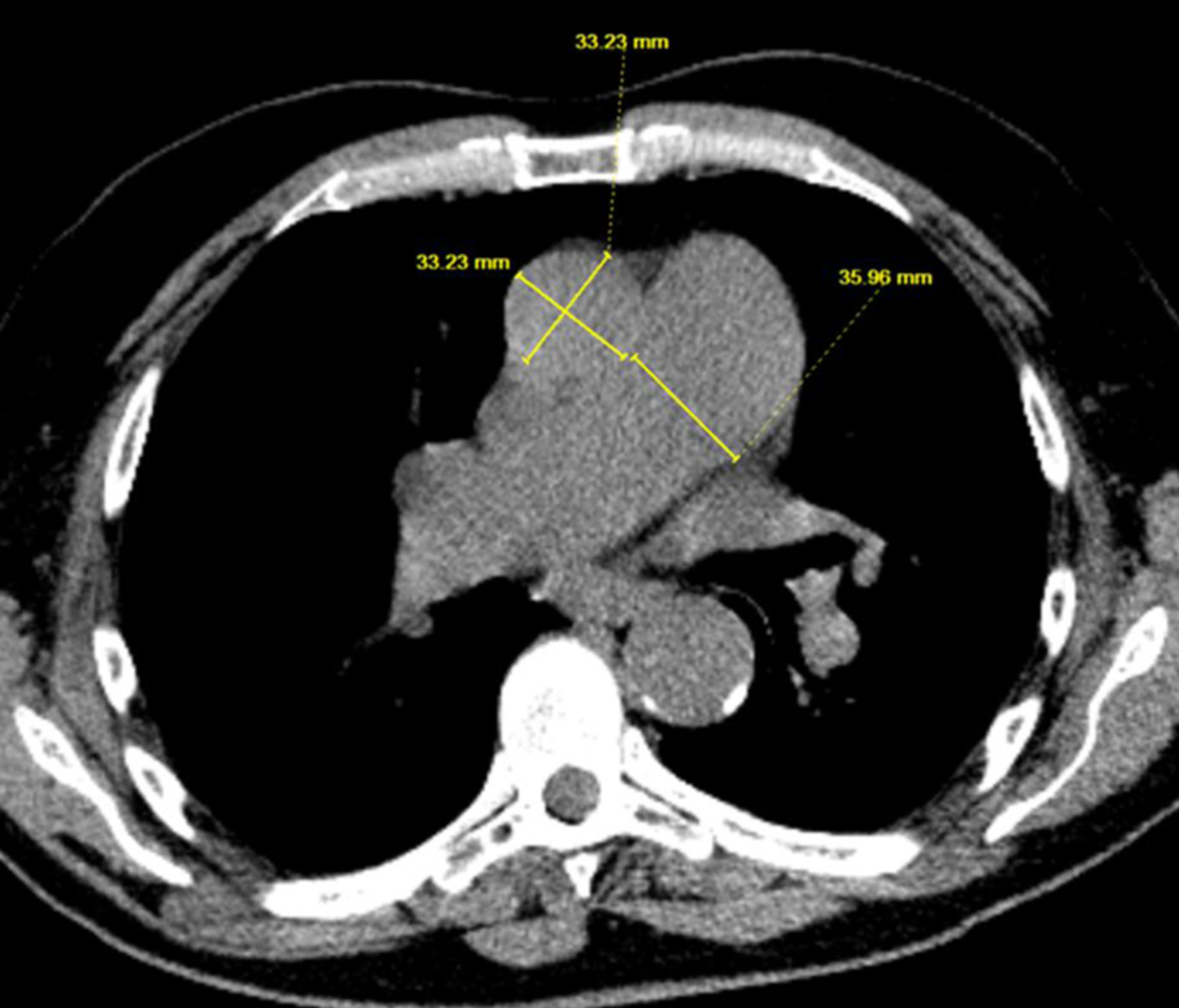 Click for large image | Figure 1. Imaging showing PAD and AoD after being on immunotherapy for 16 months. AoD: aorta diameter; PAD: pulmonary artery diameter. |
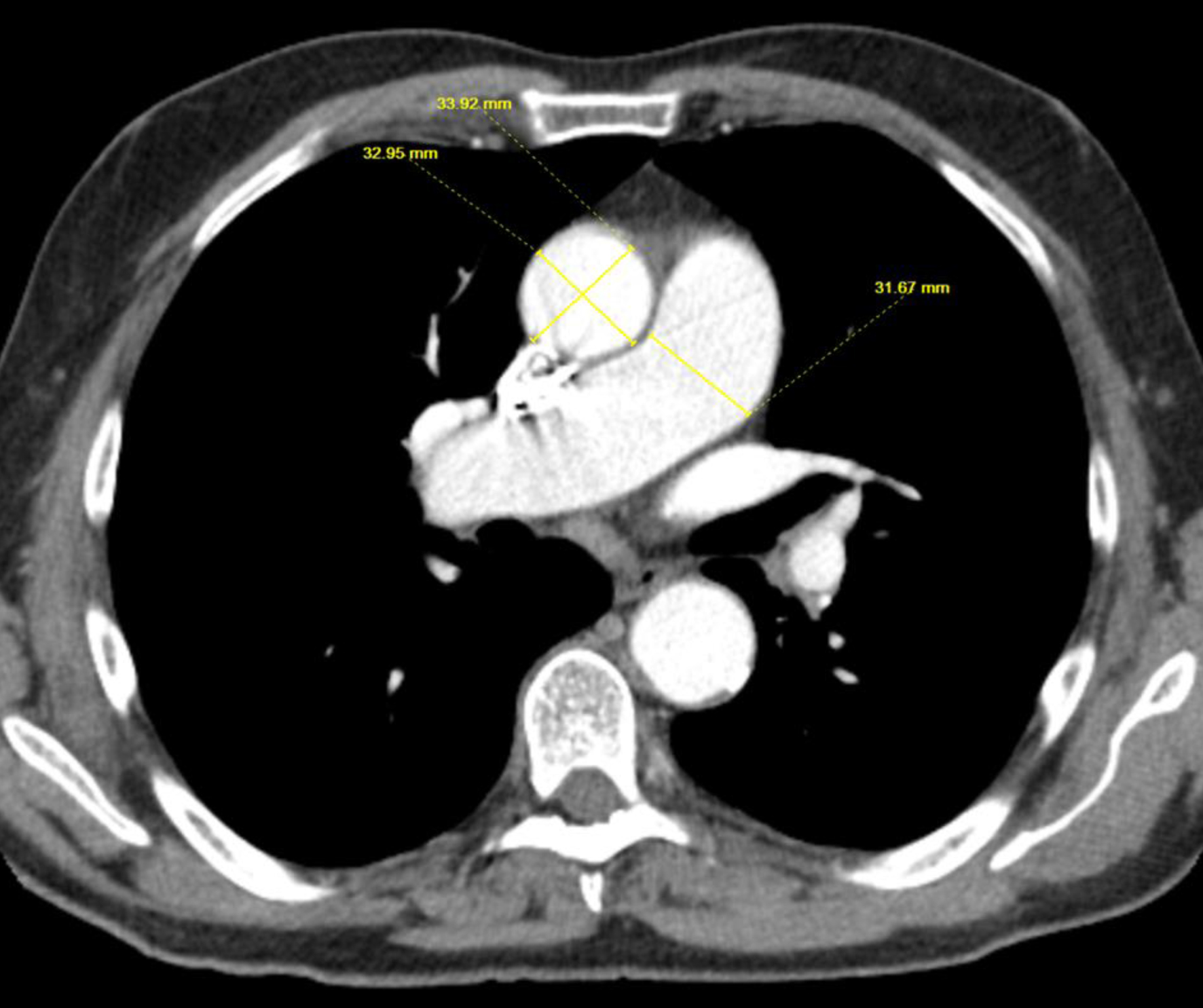 Click for large image | Figure 2. Image demonstrating PAD and AoD prior to initiation of immunotherapy. AoD: aorta diameter; PAD: pulmonary artery diameter. |
| Discussion | ▴Top |
Pembrolizumab is an ICI that targets the PD-1 pathway, thereby activating T cells to attack cancer cells [2]. ICI-induced PAH is rare and the exact mechanism is unclear [2]. Diagnosis of PAH in cancer patients on immunotherapy can increase the mortality and morbidity [2]. Most of the cases diagnosed in the literature are based on diagnosis of exclusion as we do not have specific guidelines to diagnose and manage ICI-induced PAH [1]. This can lead to delayed diagnosis and can result in poor patient outcomes [1]. Pattern of PAH caused by ICI is through endothelial injury, thereby leading to group 1 precapillary type like in our patient [4].
Endothelial injury is more likely mediated by exaggerated immune response caused by immunotherapy leading to production of oxygen-free radicals causing tissue damage, endothelial dysfunction, and vasculitis [4]. Increased inflammation due to vasculitis leads to the loss of balance between vasoconstriction and vasodilation, thereby increasing the stiffness of pulmonary vasculature causing PAH [4].
In a retrospective study conducted by Palassin et al reviewing pharmacovigilance databases looking at PAH reported with ICI, they found 42 PAH cases among 73,032 ICI-associated adverse events with a fatality rate of 31% [1]. This shows the rarity and severity of PAH caused by ICI [1]. Among them, 50% are lung cancer patients and 22.2% are diagnosed with melanoma, with around 50% of them receiving nivolumab followed by pembrolizumab (14.3%) [1]. The median time to onset of symptoms of PAH was 77 days after the ICI treatment initiation [1]. Among patients diagnosed with PAH receiving ICI, 45% did not recover, 18% were recovering, and less than 10% patients recovered per their report [1]. The more likely explanation of high fatality rate is the failure to diagnose it early and no standard treatment guidelines available to treat ICI-mediated PAH [1]. This signifies the importance of formulating guidelines for accurate diagnosis and timely treatment of PAH in this high-risk patient population [5]. Studies have shown that presence of a tumor will increase the expression of PD-L1 on cells, inhibition of which can promote the occurrence of more side effects at target organs [1]. So, there is a high likelihood of pulmonary toxicity in lung cancer patients compared to other solid tumors and these patients should be monitored closely compared to other solid tumors [1].
In another retrospective study conducted by Mylvaganam et al looking at the impact of ICI on pulmonary vasculature and right ventricular function, they found that ICI is associated with increased median PAD and AoD ratio from 0.83 to 0.89 (P = 0.03) and increased right ventricular free wall longitudinal systolic strain after a median exposure of 59 and 85 days, respectively [6]. There shows that ICI does have an impact on pulmonary vasculature and RV and severity might vary based on patient comorbidities [5].
Literature review has shown that PAD and PAD:AoD ratio is helpful in diagnosing PAH early [5]. In a retrospective study conducted by Zhao et al on lung cancer patients receiving nivolumab, they found that mean PAD has increased from 26.3 ± 2.8 mm versus 28.0 ± 3.0 mm (P < 0.001) pre- and post-treatment respectively and two patients had life-threatening complications due to PAH, both showing post-treatment PAD:AoD ratio > 1 [4]. So, these studies reinforce the significance of monitoring computed tomography (CT) scan and ECHO findings to closely monitor PAD and AoD to identify early cases of PAH to decrease morbidity and mortality [4]. More research needs to be done to find out which factors contribute to the PAH progression and its severity [4].
Apart from discontinuing ICI, no specific guidelines exist for management of ICI-induced PAH. Our patient got started on riociguat and macitentan. Riociguat is a guanylate cyclase stimulator and has been known to improve PVR, exercise tolerance, and brain natriuretic peptide (BNP) levels [7]. Macitentan is an endothelin receptor antagonist approved by Food and Drug Administration (FDA) for PAH [8]. Most of the tumors secrete excess fibrous tissue through fibroblasts to prevent the entry of T cells, thereby creating an immune-excluded tumor environment [8]. In a study conducted by Son et al where they created macitentan nanoparticles and repositioned them in the tumor microenvironment (TME), they found that these nanoparticles regulate the expression of fibroblasts and decrease fibrotic progression of tumor cells and also decrease the activity of cancer cells derived exosomes, thus modulating the TME and regulatory T (Treg) cells and maintaining good balance between ICI-mediated tumor response and its toxicity [8]. Interleukin (IL)-12 and IL-17 also play a role in inflammatory-mediated PAH and B-cell depletion with rituximab has been known to decrease IL-12 and IL-17 levels predictive of favorable response to the drug [9]. More studies are needed to explore the role of rituximab and other immunosuppressive agents in early treatment of PAH to avoid further vascular damage [9].
Imbalance in gut bacteria composition is known to happen in cancer patients. This allows increased pathogenic bacterial product translocation from the gut into systemic circulation and increases trimethyl N-oxide and serotonin [10]. This aggravates peripulmonary vascular inflammation, endothelial dysfunction, and worsening of PAH [10]. This raises a question to see if there is any role of fecal microbiota transplantation (FMT) where functional bacteria from healthy donor feces is transferred to patients to repair gut dysbiosis in improving outcomes in PAH [10]. To answer this question, there is a clinical trial being conducted to evaluate the efficacy of FMT in treating PAH [11]. It might be hard to conduct a specific study looking at the role of FMT in treating ICI-induced PAH due to the small number of patients, but if the above study shows positive results, it is worth applying those results to treat ICI-induced PAH.
Conclusion
PAH due to ICI is a less common side effect with high fatality rate. Most of the time, it is a diagnosis of exclusion and given the rarity of the disease, no specific diagnostic and treatment guidelines exist. Early detection by closely monitoring PAD and AoD to identify PAH in patients receiving ICI is essential to avoid severe cardiovascular complications. Apart from vasodilators, the role of immunosuppression such as steroids and rituximab needs to be explored. More studies are needed to determine the role of FMT in treating PAH due to ICI.
Acknowledgments
None to declare.
Financial Disclosure
The authors declare that they do not have a financial relationship with any commercial entity that has an interest in the subject of this manuscript.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Informed Consent
Informed consent has been obtained.
Author Contributions
Lakshmi Manogna Chintalacheruvu: corresponding author, helped in framing the concept, diagnosis management, literature review, editing, and primary manuscript drafting. Vamsi Krishna Chilluru: data collection literature review and manuscript editing. Narendra Babu Gutta: radiological analysis image preparation and manuscript editing. All authors reviewed and approved final manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
AoD: aorta diameter; BNP: brain natriuretic peptide; CT: computed tomography; CTLA-4: cytotoxic T-lymphocyte-associated antigen 4; ECHO: echocardiography; FDA: Food and Drug Administration; FDG: F-fluorodeoxyglucose; FMT: fecal microbiota transplantation; ICIs: immune checkpoint inhibitors; LN: lymph node; MRI: magnetic resonance imaging; NGS: next-generation sequencing; PAD: pulmonary artery diameter; PAH: pulmonary arterial hypertension; PD-1: programmed cell death-1; PD-L1: program cell death-ligand 1; PASP: pulmonary artery systolic pressure; PET: positron emission tomography; RV: right ventricle; RVSP: right ventricular systolic pressure; SOB: shortness of breath; TMB: tumor mutational burden; TME: tumor microenvironment
| References | ▴Top |
- Palassin P, Maria ATJ, Ladhari C, Quantin X, Montani D, Bourdin A, Boissin C, et al. Pulmonary hypertension reported with immune checkpoint inhibitors: a pharmacovigilance study. Cancer Immunol Immunother. 2022;71(12):3093-3097.
doi pubmed - Leiva O, Beaty W, Soo S, Agarwal MA, Yang EH. Cancer therapy-associated pulmonary hypertension and right ventricular dysfunction: etiologies and prognostic implications. Rev Cardiovasc Med. 2024;25(3):87.
doi pubmed - Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018-2028.
doi pubmed - Zhao H, Song J, Li X, Xia Z, Wang Q, Fu J, Miao Y, et al. The role of immune cells and inflammation in pulmonary hypertension: mechanisms and implications. Front Immunol. 2024;15:1374506.
doi pubmed - Shen Y, Wan C, Tian P, Wu Y, Li X, Yang T, An J, et al. CT-base pulmonary artery measurement in the detection of pulmonary hypertension: a meta-analysis and systematic review. Medicine (Baltimore). 2014;93(27):e256.
doi pubmed - Mylvaganam R, Avery R, Goldberg I, Makowski C, Kalhan R, Villaflor V, Cuttica MJ. Adverse effects of immune checkpoint inhibitor therapies on right ventricular function and pulmonary arterial dilatation. Pulm Circ. 2021;11(1):2045894021992236.
doi pubmed - Ghofrani HA, Galie N, Grimminger F, Grunig E, Humbert M, Jing ZC, Keogh AM, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369(4):330-340.
doi pubmed - Son S, Shin JM, Shin S, Kim CH, Lee JA, Ko H, Lee ES, et al. Repurposing macitentan with nanoparticle modulates tumor microenvironment to potentiate immune checkpoint blockade. Biomaterials. 2021;276:121058.
doi pubmed - Zamanian RT, Badesch D, Chung L, Domsic RT, Medsger T, Pinckney A, Keyes-Elstein L, et al. Safety and efficacy of B-cell depletion with rituximab for the treatment of systemic sclerosis-associated pulmonary arterial hypertension: a multicenter, double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2021;204(2):209-221.
doi pubmed - Chen YH, Yuan W, Meng LK, Zhong JC, Liu XY. The role and mechanism of gut microbiota in pulmonary arterial hypertension. Nutrients. 2022;14(20):4278.
doi pubmed - Moutsoglou DM. 2021 American thoracic society BEAR cage winning proposal: microbiome transplant in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2022;205(1):13-16.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.