| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com |
Case Report
Volume 16, Number 2, February 2025, pages 87-93
A Peculiar Case of Fetal Distress Leading to the Diagnosis of Diabetic Ketoacidosis in Pregnancy
Wen Yu Quaka, d, Zhi Wen Lohb, Poh Ting Lima, Wai Kheong Ryan Leec
aDepartment of Obstetrics and Gynecology, KK Women’s and Children’s Hospital, Singapore 229899, Singapore
bDepartment of Endocrinology, Tan Tock Seng Hospital, Singapore, Singapore
cDepartment of Maternal Fetal Medicine, KK Women’s and Children’s Hospital, Singapore, Singapore
dCorresponding Author: Wen Yu Quak, Department of Obstetrics and Gynecology, KK Women’s and Children’s Hospital, Singapore 229899, Singapore
Manuscript submitted December 2, 2024, accepted January 23, 2025, published online February 2, 2025
Short title: Fetal Distress From Diabetic Ketoacidosis
doi: https://doi.org/10.14740/jmc5088
| Abstract | ▴Top |
Diabetes ketoacidosis (DKA) in pregnancy is associated with significant maternal and neonatal morbidity. It is rare for women without a prior history of diabetes mellitus (DM) to develop DKA. This case report describes an atypical presentation of DKA in a 38-year-old primigravida, with no history of DM, presenting with “unexplained” fetal distress. She presented at 25 weeks to our labor ward triage with an unrelated complaint of prolapsed piles. There were no complaints of reduced fetal movement, abdominal or contraction pains or per vaginal bleeding. Ultrasonography showed an appropriately grown fetus with normal liquor volume. Incidental fetal distress was picked up on a cardiotocography (CTG) which showed a fetal heart rate of 150 beats per minute with reduced variability and shallow decelerations. The unlikely diagnosis of DKA was suspected when a random capillary blood glucose (CBG) level returned as “HI”. Investigations revealed the triad of elevated venous glucose, raised serum ketones and high anion gap metabolic acidosis (with a maternal pH of 7.14), consistent with the diagnosis of DKA. She was aggressively treated with intravenous insulin and hydration therapy. Fetal distress resolved with resolution of the DKA. She eventually delivered a healthy baby at 37-week gestation. This case raises awareness of a rare occurrence of DKA in late pregnancy as the first presentation of DM and highlights the importance of considering a hyperglycemic crisis as a potential cause of a suspicious CTG in an asymptomatic woman without any clear reason for fetal distress. Timely diagnosis and prompt treatment of the underlying condition is lifesaving, and avoids urgent delivery and risks associated with prematurity.
Keywords: Diabetes ketoacidosis; Fetal distress; Pregnancy; Non-reassuring fetal status
| Introduction | ▴Top |
Diabetic ketoacidosis (DKA) in pregnancy is an uncommon obstetrics and medical emergency that can lead to maternal and fetal compromise [1]. The incidence is estimated to be approximately 0.5-3% among women with pre-existing diabetes mellitus (DM) [2-5]. Maternal mortality is rare, but fetal mortality is reported to be approximately 15% [6]. In pregnancies that are continued, DKA is associated with increased risk of premature delivery and neonatal intensive care unit (NICU) admissions [2, 6].
Pregnancy-related hormone changes, an altered acid-base equilibrium and an increased metabolic demand predispose pregnant women to developing DKA [7].
DKA as the first presentation of type 1 diabetes mellitus (T1DM) occurs most frequently in young children. Common presenting symptoms include abdominal pain, polyuria, polydipsia, weight loss, fatigue, or altered mental status. The occurrence of DKA in pregnancy is most commonly associated in patients with T1DM, and rarely in patients with type 2 diabetes mellitus (T2DM) or gestational diabetes mellitus (GDM) [8].
This case report details our experience in the diagnosis and management of a peculiar case of DKA in pregnancy as the first presentation of DM, presenting with an incidental finding of fetal distress in an otherwise asymptomatic patient.
| Case Report | ▴Top |
A 38-year-old primigravida lady presented to our labor ward at 25 weeks of gestation complaining of prolapsed hemorrhoids and constipation. She reported normal fetal movements and did not complain of abdominal pain, leaking liquor or per vaginal bleeding.
She had a past medical history of asthma, which was well controlled during her pregnancy. A health screening performed a year prior to conception was negative for DM or hyperlipidemia. There was no history of smoking, alcoholic consumption, or illicit drug use. Her only significant family history was of DM in both parents. She had made her booking early at 8 weeks, and her pregnancy had been uncomplicated. She did not pursue aneuploidy screening. There were no fetal anomalies on her 20-week screening scan.
On physical examination, she appeared well and comfortable. Her vitals were stable, and she was afebrile. Her blood pressure measured 129/89 mm Hg, and her respiratory rate was 22 breaths per minute. Abdominal examination did not reveal any tenderness or palpable uterine contractions. The vaginal examination was unremarkable and there was no vaginal bleeding or liquor seen. Her cervix was not dilated. The fetal fibronectin test performed was negative. Transabdominal ultrasonography showed an appropriately grown fetus with bio-parameters in keeping with 25-week gestation and a normal amniotic fluid index.
However, a routine cardiotocography (CTG) performed revealed an incidental finding of fetal distress with a pathological trace [9]. The baseline fetal heart rate was 150 beats per minute with reduced fetal variability and repetitive decelerations (Fig. 1).
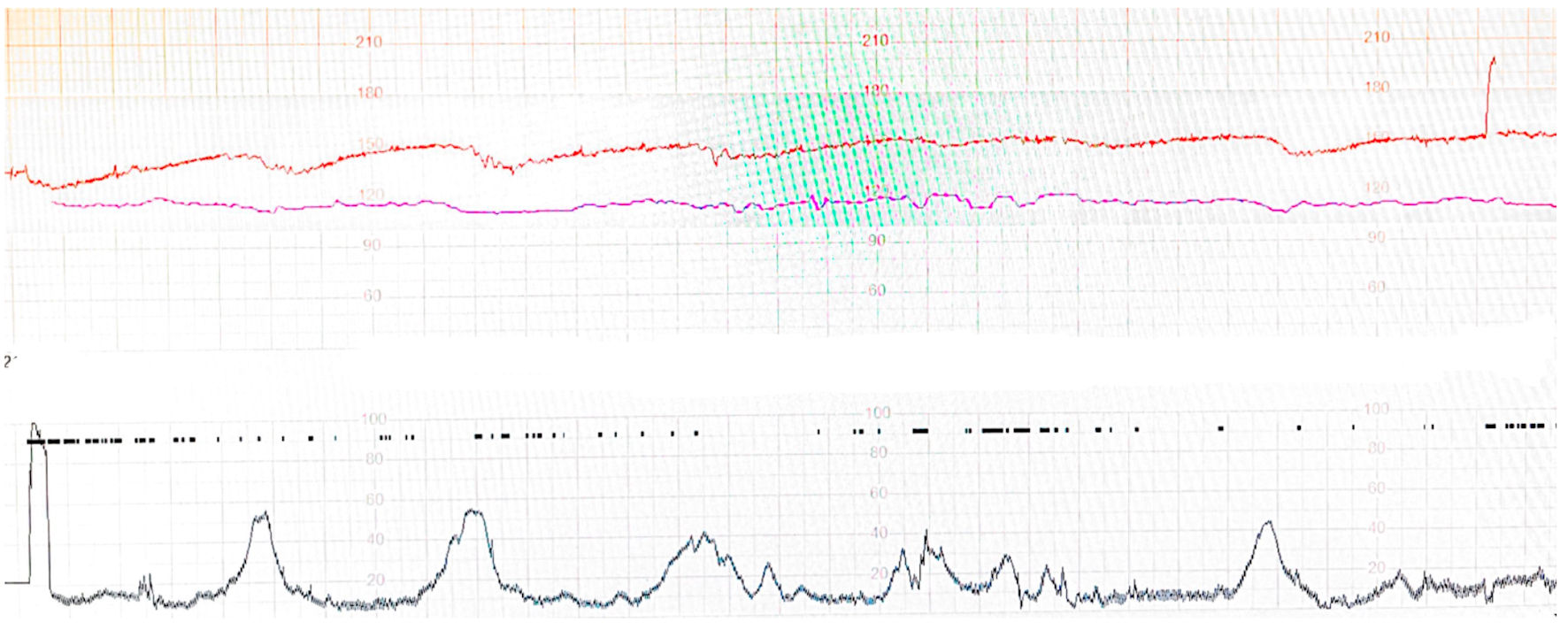 Click for large image | Figure 1. Fetal cardiotocography of the patient at initial presentation showing reduced variability and decelerations. |
With her normal examination findings and relatively benign presenting complaint, there was no discernible cause for the fetal distress on initial clinical assessment. She was immediately admitted to the labor ward for continuous CTG monitoring and given intravenous (IV) hydration. Intramuscular dexamethasone was given for fetal lung maturity in anticipation for premature delivery, should fetal distress persist.
Investigations
Various blood tests were performed to investigate for causes of fetal distress. A random capillary blood glucose (CBG) was performed and returned as “HI”. Further blood tests revealed the classic triad of elevated venous glucose, raised ketones and high anion gap metabolic acidosis (HAGMA), confirming the diagnosis of DKA (Table 1).
 Click to view | Table 1. Summary of Blood Investigation Results at Initial Presentation |
Of note, the glycated hemoglobin (HbA1c) was > 14.0%. Effective serum osmolality (2 (Na+) + glucose) was 284 mmol/L (reference index 285 - 295 mmol/L). This makes the diagnosis of hyperosmolar hyperglycemic state less likely, as serum osmolality is less than 320 mmol/L. Assuming a serum bicarbonate reading of 5 mmol/L, the partial pressure of CO2 (pCO2) result was reflective of appropriate respiratory compensation for metabolic acidosis when calculated using Winter’s formula (expected pCO2: 15.5 mm Hg).
The hyperglycemia contributed to the moderate hyponatremia in her case. The serum sodium was 137 mmol/L when corrected for glucose (using Hillier’s formula). The high-normal serum potassium and high phosphate levels were also explained by a shift of both electrolytes from the intra to extra-cellular space driven by insulin deficiency, hypertonicity, and acidosis [1, 2]. Moderately severe fasting hypertriglyceridemia was present. Lactate was normal at 1.4 mmol/L (reference index 0.5 - 2.2 mmol/L). She had no clinical or biochemical suggestions of sepsis.
A 12-lead electrocardiogram revealed sinus tachycardia at 120 beats per minute with no ST-T changes to suggest ischemia.
Anti-glutamic acid decarboxylase and anti-islet cell antibodies were subsequently tested to assess for T1DM and were negative.
Diagnosis
A diagnosis of DKA was established, with a pathological CTG tracing reflecting concomitant fetal acidosis.
Treatment
She was consequently managed in an obstetrics high dependency unit under the care of a multidisciplinary team involving specialists from the high-risk maternal fetal medicine team, neonatology, and endocrinology. Close monitoring of the patient’s mental status and hemodynamic trend was immediately instituted. Strict intake-output charting was aided by an indwelling urinary catheter. Adequate vascular access was ensured with three large-bore IV cannulas, and hourly CBG monitoring was commenced.
In discussion with our maternal fetal medicine specialists and neonatologists, an initial plan for cesarean delivery for persistent fetal distress was deferred in view of the risks of extreme fetal prematurity, at 25-week gestation, and to focus on aggressive treatment of maternal acidosis. Close fetal surveillance was instituted with continuous CTG monitoring, with a plan for delivery, should fetal distress have persisted despite DKA treatment.
IV hydration was started with normal saline infusion. The estimated fluid deficit was 100 mL/kg body weight [4], which translates to 4,600 mL in our patient. Initial fluid resuscitation adhered to guideline recommendations, with normal saline infused at a rate of 1 L over the first hour, then 1 L over the next 2 h, and 1 L over the following 4 h. Improvement in fluid status was evidenced by an adequate hourly urine output volume ranging between 50 and -120 mL, well above the threshold of 0.5 mL/kg/h. A 3-L normal saline maintenance drip followed this to replace the estimated fluid deficit within the first 24 h of treatment [1, 2]. Dextrose was added to the drip when CBG fell below 11.1 mmol/L.
Regular insulin was administered intravenously as a bolus of 5 units (approximately 0.1 units/kg), immediately followed by a variable rate infusion starting at 5 units/h. The hourly insulin dose was increased by 1 unit/h when the target CBG reduction of 3 mmol/L/h was not met. The trend of hourly CBG readings with corresponding insulin doses are depicted in Figure 2.
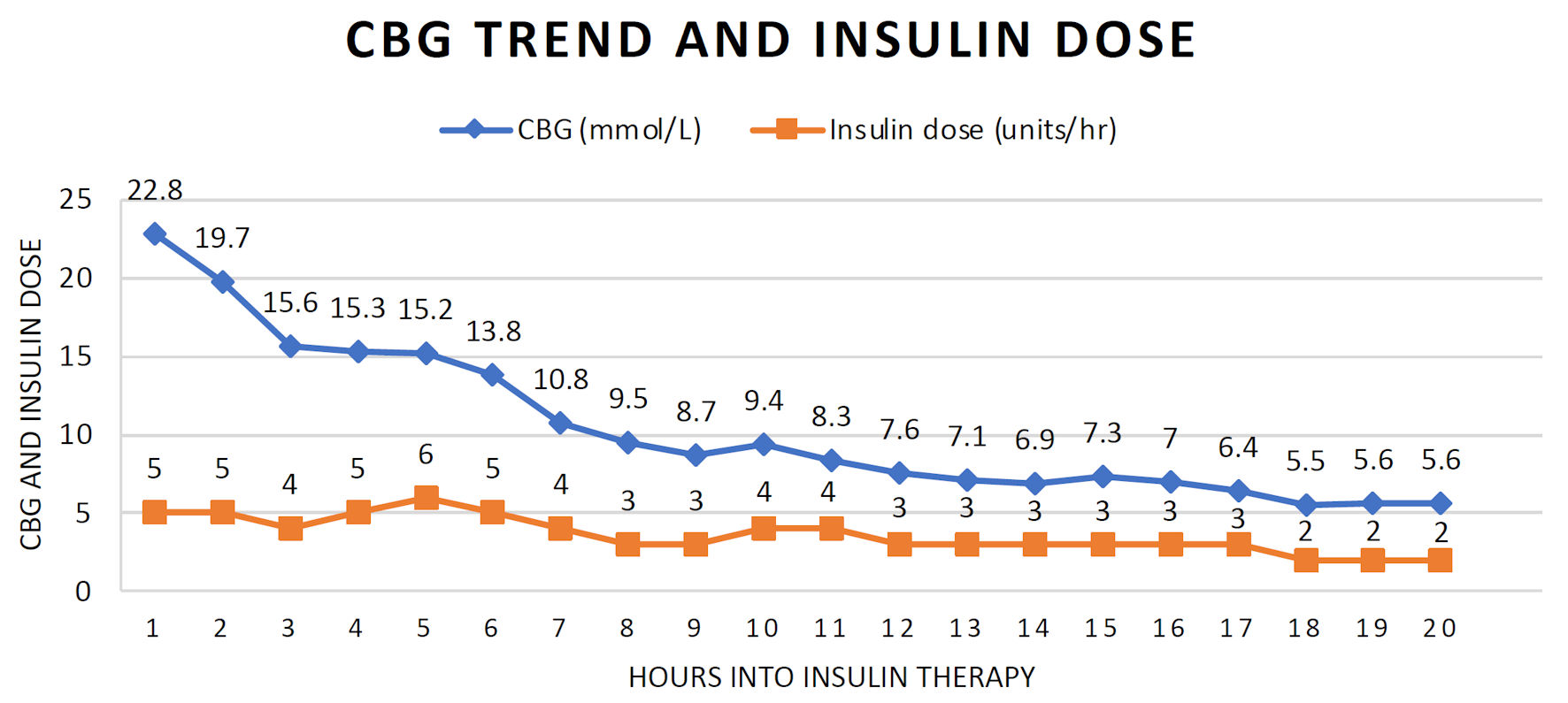 Click for large image | Figure 2. Capillary blood glucose trend and intravenous insulin dose during treatment. |
The treatment was successful, and a reactive fetal trace was observed on CTG upon clearance of ketonemia. The patient was transited to subcutaneous (SC) insulin 20 h into treatment. IV insulin was ceased 2 h after SC insulin was administered.
The patient was subsequently transferred to the general ward for continued inpatient management for newly diagnosed DM in pregnancy. She was seen by the diabetic nurse clinician and dietician for further advice on home glucose monitoring and diet control. Upon optimization of her glucose levels, she was discharged on a basal-bolus insulin regime with outpatient home glucose monitoring.
Follow-up and outcomes
The patient was closely monitored in the outpatient clinic with follow-up visits every 2 weeks. Ongoing care was provided by the high-risk maternal obstetrics team in collaboration with endocrinology specialists.
Home CBG readings were largely within the acceptable fasting (< 5.3mmol/L) and 2 h post-prandial (< 6.7mmol/L) cut-offs [10], without any hypoglycemia symptoms or readings. With marked improvement in her glycemic control, HbA1c improved to 6.0% by 36 weeks of pregnancy. Fetal growth parameters remained within normal range.
She eventually delivered at 37-weeks of gestation via elective lower segment cesarean section for breech presentation, having declined the option of an external cephalic version. She gave birth to a healthy baby boy with a birth weight of 3.4 kg.
On the morning of surgery, a variable rate of IV insulin infusion at 1 unit/h, paired with a dextrose saline drip at 2 L/day, was started as per hospital protocol. CBG ranged between 3.9 and 4.4 mmol/L up until delivery of the baby, with the IV insulin dose promptly halved to 0.5 units/h thereafter.
Given the tight inpatient CBG trend and expected decrease in insulin requirement after delivery, a reduced dose of SC detemir at 4 units was used to facilitate the transition from IV insulin. SC NovoRapid was re-started at 4 units together with diet. She experienced asymptomatic hypoglycemia at bedtime despite good oral intake. As a result, NovoRapid was halved further to 2 units thrice daily with detemir continued at 4 units twice daily. Her CBG over the rest of the admission ranged between 4.1 and 9.8 mmol/L.
There was no episode of neonatal hypoglycemia, and both mother and baby were discharged well on day 2 postnatally.
| Discussion | ▴Top |
This case report describes an atypical presentation of DKA in pregnancy, presenting with incidental fetal distress at 25-week gestation, in an otherwise asymptomatic patient with no history of DM. Maternal ketonemia and fetal distress resolved with prompt multidisciplinary treatment, resulting in a healthy term delivery.
The incidence of DKA in pregnancy is reported to be approximately 0.5-3% [2-5]. A retrospective study performed by Grasch et al [1] revealed that among 95 women diagnosed with DKA in pregnancy between 2012 to 2021, 75 (77.3%) had type 1 diabetes and only one (1.1%) unbooked patient had no previous history of DM. Median gestational age at presentation of DKA was 29 weeks, and common precipitating factors include gastrointestinal illness or vomiting (38.0%), infection (25.6%), and insulin nonadherence (20.9%) [1]. In 2023, Xu et al presented a case series of three patients with normal glucose tolerance test during routine gestational diabetes screening, who were later diagnosed with DKA in late pregnancy [11]. All three women had significant symptoms such as polyuria, polydipsia, dizziness or vomiting. Two out of the three patients had a stillbirth. In our case report, we present a rare case of DKA presenting for the first time in late gestation in an asymptomatic patient with no known history of diabetes.
According to the Joint British Diabetes Societies Inpatient Care Group guidelines, the diagnosis of DKA in pregnancy is defined as blood ketone level more than or equal to 3.0 mmol/L (or) urine ketone level more than 2+, blood glucose level more than 11.0 mmol/L or known DM, and a bicarbonate level of less than 15 mmol/L and/or venous pH less than 7.3 [12]. This glucose level cut-off is slightly lower than the DKA diagnostic criteria for non-pregnant patients of 13.9 mmol/L [13]. This is likely because pregnancy is associated with physiological changes that can predispose women to DKA even at lower glycemic levels [7].
Insulin sensitivity drops by 56% by 36 weeks of gestation. This is the result of hormonal changes such as increased estrogen, progesterone, cortisol and human placental lactogen. As such, pregnancy is considered an insulin-resistant state, and in addition to a state of respiratory alkalosis contributed by from a reduction in functional lung capacity and hyperventilation, pregnant women are at greater risk of DKA compared to their non-pregnant counterparts [14, 15]. In fact, a study performed by Awwad et al investigated the effect of maternal fasting during Ramadan and found that the rate of ketosis was higher in fasted pregnant women (11.3% vs 5.0%) [16].
Ketoacids and glucose readily cross the placenta [17]. This creates an equilibrium state between the fetal and maternal circulation, in which the fetus begins to reflect the maternal acidotic state in a diabetic crisis. Depressed sympathetic and parasympathetic activity from the ensuring fetal ketosis and acidotic state would then “present” on the cardiotocograph with reduced variability, as we observed in this case. Hence, it is important to have a high index of suspicion for DKA or other causes for metabolic acidosis if there are no obvious obstetric factors causing a pathological CTG trace reflecting fetal acidosis.
While it is tempting to deliver a patient based on a pathological CTG tracing, one has to balance the risks of fetal prematurity and the risks of fetal morbidity in utero. Close fetal surveillance is recommended for patients undergoing treatment for DKA. Initial CTG tracing may reflect fetal acidotic state with reduced variability or late decelerations, but studies show that these changes are reversible with hydration and correction of maternal acidosis [18]. Normalization of the fetal heart tracing may require 4 - 8 h after resolution of DKA [8]. Decision to deliver should be individualized and made by a multidisciplinary team with experts from the high-risk obstetrics team, neonatology and endocrinology, after evaluating for maternal clinical status, fetal gestational age and wellbeing.
The management of DKA comprises intravascular volume repletion, clearance of ketoacidosis, control of hyperglycemia, correction of electrolyte imbalances, and identification and treatment of precipitants. Intravenous hydration restores circulatory volume, enhances tissue perfusion, and reduces counter-regulatory hormone levels. This facilitates increased insulin sensitivity and improves hyperglycemia. Insulin controls hyperglycemia by decreasing hepatic glucose production and enhancing peripheral utilization. It also promotes clearance of ketonemia by reducing lipolysis and glucagon secretion [2-4].
High insulin resistance contributed by the pregnancy had to be overcome. The waning effect of dexamethasone during the treatment course would also influence insulin requirement. Mild metabolic acidosis (as compensation for respiratory alkalosis) representing altered physiology in pregnancy could complicate the assessment of DKA resolution. Potassium is driven intracellularly by insulin therapy and correction of acidosis. Hypokalemia is common and can lead to cardiac arrhythmias especially when severe. Hence, attention must be paid to ensure adequate potassium replacement during insulin treatment.
Attempting to accurately categorize the type of DM in our patient at the time of presentation was challenging. DKA is the diabetic emergency typical of T1DM, which reflects absolute insulin deficiency. Our patient’s normal body mass index (BMI) and absence of features to suggest insulin resistance or metabolic syndrome strengthened this possibility. A positive autoantibody screen would have clinched the diagnosis of T1DM. However, it was negative in her case, and is so, for at least 10-15% of individuals with type 1 DM [19].
T2DM accounts for the large majority of between 90% and 95% of the population with DM [20]. The positive family history and negative autoantibody profile support this diagnosis. In addition, marked insulin resistance, typical of T2DM and especially in the later stages of pregnancy, was evident, with the total daily insulin dose of 1 unit/kg in the third trimester. Though infrequent, ketoacidosis may develop in stress, which for our patient would be the combination of advanced pregnancy and high-dose glucocorticoid use.
GDM remained possible. She first presented with hyperglycemia during the second to third trimester of pregnancy, which matches the timeline described [20]. However, literature suggests it is unusual for GDM to manifest as DKA [21, 22].
The markedly elevated HbA1c result prompted further consideration regarding the onset of hyperglycemia. HbA1c reflects the cumulative glucose exposure of erythrocytes over a preceding time frame proportional to erythrocyte survival (about 90 - 120 days), with plasma glucose concentrations of the most recent 4 weeks having greatest influence [23]. Though this implies a degree of chronicity, for our patient it does not clearly differentiate between pre-gestational DM (type 1 and type 2 DM) or GDM.
The precise classification of diabetes is important in determining therapy. Given that T1DM was a consideration, she was managed accordingly with basal bolus insulin regime throughout pregnancy and continued as such, albeit with reduced doses, post-delivery.
She was well when reviewed in the endocrinology clinic 8 weeks post-delivery. She had been adherent to multi-dose subcutaneous insulin regime of detemir 4 units twice daily and NovoRapid 2 units thrice daily with meals.
Overall control of hyperglycemia remained optimal, with HbA1c level within the ideal range at 6.2%. A normal fasting glucose at 5.2 mmol/L was paired with an elevated venous glucose at 17.6 mmol/L (after ingestion of 75 g of glucose) on the oral glucose tolerance test, which suggests persistence of DM beyond pregnancy, hence ruling out the differential of GDM.
The robust fasting C-peptide result of 1,072 pmol/L (reference index 364 - 1,655 pmol/L) supports the presence endogenous insulin production; hence the diagnosis aligns more with T2DM. Absolute insulin deficiency, which is the hallmark of T1DM, would be characterized by a C-peptide typically < 200 pmol/L [24].
The heterogeneous nature of DM subtypes, together with her atypical presentation, made the classification of DM challenging in our patient. It may not be possible to definitively subtype an individual at the time of presentation, and accurate classification may only become possible over time.
Conclusions
Antepartum fetal distress, in the absence of obvious obstetric or fetal causes may suggest maternal compromise even in the absence of significant symptoms. Diabetic ketoacidosis is an uncommon medical emergency in pregnancy, with significant fetal morbidity and mortality risk. Hence, it is imperative to keep a high index of suspicion for this rare differential in cases of “unexplained” fetal distress. Care for such patients should be undertaken by a multidisciplinary team involving maternal medicine specialists, neonatologists and endocrinologists to optimize maternal and fetal outcomes. In a preterm infant, the benefits of delivery must be weighed against the risks of prematurity. Early identification and correction of the underlying maternal pathology may avoid unnecessary delivery in these infants.
Learning points
Fetal well-being can be a reflection of maternal health. Fetal distress, in the absence of fetal factors such as structural anomaly, growth restriction or oligohydramnios, should prompt clinicians to assess maternal well-being and potential compromise from medical emergencies, such as DKA.
In a preterm infant, the benefits of delivery must be weighed against the risks of prematurity. Early identification and correction of the underlying maternal pathology may avoid unnecessary iatrogenic deliveries in these infants.
Diabetic ketoacidosis in pregnancy is a rare, but life-threatening medical emergency associated with adverse maternal and fetal outcomes.
Rapid and aggressive treatment of DKA is critical to avoid maternal and fetal morbidity or mortality. A combined multidisciplinary care involving maternal fetal medicine specialists, endocrinology and diabetic specialist nurses is critical to ensure safe passage of both mother and fetus through the course of pregnancy.
Accurate classification of DM in pregnancy, while critical for tailoring treatment, can be challenging in the acute setting, particularly in cases with atypical presentations. Long-term follow-up is necessary to allow for a concise diagnosis to be made and to optimize post-natal management.
Acknowledgments
None to declare.
Financial Disclosure
There is no funding required for this study.
Conflict of Interest
There is no conflict of interest to disclose.
Informed Consent
Informed consent was obtained from the patient for publication of this case report.
Author Contributions
Quak WY was the resident involved in the care and diagnosis of the patient, as well as the corresponding author and the main writer of the manuscript. Loh ZW was the endocrinologist involved in the care of the patient and involved in the writing of the manuscript. Lim PT performed literature review and was involved in writing the manuscript. Lee WK Ryan conceptualized, supervised and edited this case report.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Grasch JL, Lammers S, Scaglia Drusini F, Vickery SS, Venkatesh KK, Thung S, McKiever ME, et al. Clinical presentation and outcomes of diabetic ketoacidosis in pregnancy. Obstet Gynecol. 2024;144(5):590-598.
doi pubmed - Sibai BM, Viteri OA. Diabetic ketoacidosis in pregnancy. Obstet Gynecol. 2014;123(1):167-178.
doi pubmed - Hawthorne G. Maternal complications in diabetic pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(1):77-90.
doi pubmed - Ramin KD. Diabetic ketoacidosis in pregnancy. Obstet Gynecol Clin North Am. 1999;26(3):481-488.
doi pubmed - Chauhan SP, Perry KG, Jr., McLaughlin BN, Roberts WE, Sullivan CA, Morrison JC. Diabetic ketoacidosis complicating pregnancy. J Perinatol. 1996;16(3 Pt 1):173-175.
pubmed - Morrison FJR, Movassaghian M, Seely EW, Curran A, Shubina M, Morton-Eggleston E, Zera CA, et al. Fetal outcomes after diabetic ketoacidosis during pregnancy. Diabetes Care. 2017;40(7):e77-e79.
doi pubmed - Maheswaran Dhanasekaran SM, Egan AM, Dhanasekaran N, Diabet EM. Diabetic ketoacidosis in pregnancy: an overview of pathophysiology, management, and pregnancy outcomes. EASD 2022. 2022:62.
- Mohan M, Baagar KAM, Lindow S. Management of diabetic ketoacidosis in pregnancy. Obstetrician & Gynaecologist. 2017;19(1):55-62.
- Ayres-de-Campos D, Spong CY, Chandraharan E, Panel FIFMEC. FIGO consensus guidelines on intrapartum fetal monitoring: Cardiotocography. Int J Gynaecol Obstet. 2015;131(1):13-24.
doi pubmed - Webber J, Charlton M, Johns N. Diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period (NG3). British Journal of Diabetes. 2015;15(3):107-111.
- Xu J, Liu C, Zhao W, Lou W. Case series of diabetic ketoacidosis in late pregnancy with normal glucose tolerance. Int J Womens Health. 2023;15:1857-1864.
doi pubmed - Savage MW, Dhatariya KK, Kilvert A, Rayman G, Rees JA, Courtney CH, Hilton L, et al. Joint British Diabetes Societies guideline for the management of diabetic ketoacidosis. Diabet Med. 2011;28(5):508-515.
doi pubmed - Trachtenbarg DE. Diabetic ketoacidosis. Am Fam Physician. 2005;71(9):1705-1714.
pubmed - Ryan EA, Enns L. Role of gestational hormones in the induction of insulin resistance. J Clin Endocrinol Metab. 1988;67(2):341-347.
doi pubmed - Kirwan JP, Hauguel-De Mouzon S, Lepercq J, Challier JC, Huston-Presley L, Friedman JE, Kalhan SC, et al. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51(7):2207-2213.
doi pubmed - Awwad J, Usta IM, Succar J, Musallam KM, Ghazeeri G, Nassar AH. The effect of maternal fasting during Ramadan on preterm delivery: a prospective cohort study. BJOG. 2012;119(11):1379-1386.
doi pubmed - Atkinson DE, Boyd RD, Sibley CP, Neill JD. Placental transfer. In Physiology of Reproduction. Elsevier BV. 2006. p. 2787-2846.
- Hagay ZJ, Weissman A, Lurie S, Insler V. Reversal of fetal distress following intensive treatment of maternal diabetic ketoacidosis. Am J Perinatol. 1994;11(6):430-432.
doi pubmed - World Health Organization. Classification of diabetes mellitus. 2019.
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S15-S33.
doi pubmed - Prior M, Gopinath D, Schram C. Gestational diabetes presenting as diabetic ketoacidosis Archives of Disease in Childhood. Fetal and Neonatal Edition. 2010;95:Fa61-Fa62.
- Maislos M, Harman-Bohem I, Weitzman S. Diabetic ketoacidosis. A rare complication of gestational diabetes. Diabetes Care. 1992;15(8):968-970.
doi pubmed - Leow MK. Glycated Hemoglobin (HbA1c): clinical applications of a mathematical concept. Acta Inform Med. 2016;24(4):233-238.
doi pubmed - Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med. 2013;30(7):803-817.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.