| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://jmc.elmerpub.com |
Case Report
Volume 16, Number 1, January 2025, pages 1-5
Anomalous Pulmonary Vein Drainage Into the Innominate Vein Diagnosed After Central Line Placement: A Case Report Along With a Review of Rare Congenital Anatomy Partial Anomalous Pulmonary Venous Return
Chris Andraosa, Sean Hormozianb, Aldin Malkocb, d, Nia Robinsonb, Michael M. Neekic, Samuel Leeb, Samuel Schwartzb, Keith Gloverb
aSchool of Medicine, St. George’s University, Grenada, West Indies
bDepartment of Surgery, Arrowhead Regional Medical Center, Colton, CA, USA
cDepartment of Emergency Medicine, Arrowhead Regional Medical Center, Colton, CA, USA
dCorresponding Author: Aldin Malkoc, Department of Surgery, Arrowhead Regional Medical Center, Colton, CA 92324, USA
Manuscript submitted August 31, 2024, accepted October 15, 2024, published online November 13, 2024
Short title: Anomalous Pulmonary Vein Drainage
doi: https://doi.org/10.14740/jmc4321
| Abstract | ▴Top |
Anomalous pulmonary vein drainage is a rare but clinically relevant variant of the cardiovascular anatomy. We present a case report of a 22-year-old male who was incidentally found to have anomalous pulmonary vein drainage into the innominate vein. The patient had a known history of seizures and was brought to the emergency department following a simple tonic-clonic seizure. He was subsequently intubated for airway protection and admitted to the medical intensive care unit (MICU). While in the MICU, a left internal jugular central venous catheter (CVC) was placed; however, post-procedural chest radiography showed the tip of the CVC in the left chest. An arterial blood gas (ABG) was concerning for arterial blood. Due to the uncertainty of line positioning, a computed tomography pulmonary angiography revealed a rare abnormal connection between the left innominate vein and the left pulmonary vein. This case underscores the importance of potential variations in pulmonary venous drainage as they may have implications for post-procedural decision-making and potentially clinical outcomes.
Keywords: Pulmonary venous system; Central line; Artery; Vascular
| Introduction | ▴Top |
The pulmonary venous system comprises a collection of four veins that deliver oxygenated blood from the lungs to the left atrium of the heart. The right superior vein drains the right superior and middle lobes of the right lung, and the right inferior vein drains the right inferior lobe of the right lung. The left superior vein drains the left superior lobe and lingula of the left lung, whereas the left inferior vein drains the left inferior lobe of the left lung [1]. Although rare, anomalous connections can occur in which the pulmonary veins connect abnormally to the systemic venous system. This can occur within intracardiac chambers or, as in this case, in extracardiac structures.
Partial anomalous pulmonary venous return (PAPVR) refers to a range of rare congenital heart defects that affects 0.4-0.7% of the population and is characterized by abnormal drainage of one or more pulmonary veins [2]. These veins do not connect to the left atrium as expected, but instead drain into the right atrium or the systemic venous system. There are reports of PAPVR involving the superior vena cava (SVC), coronary sinus, inferior vena cava (IVC), or azygous veins [3]. The following case presents a patient with a PAPVR to the left innominate vein that was discovered through post-procedural X-ray after a left internal jugular (IJ) central venous catheter (CVC) placement.
| Case Report | ▴Top |
In this case report, we describe a 22-year-old male who was incidentally found to have anomalous pulmonary vein drainage into the left innominate vein. The patient was brought to the emergency department after a suspected seizure. On arrival at the hospital, the patient exhibited symptoms of agitation, encephalopathy, and mental status changes. He was subsequently sedated and intubated due to concern for airway compromise and admitted to the medical intensive care unit (MICU). While in the MICU, the patient became hypotensive, and a CVC was placed to administer vasoactive drips. Using ultrasound guidance, the CVC was placed into the left internal jugular vein without any immediate complications. A subsequent chest radiography showed that the CVC did not cross the thoracic midline into the right atrium, but rather traveled down into the left mediastinum (Fig. 1). Given this finding, the CVC was thought to be mispositioned, and there was a concern for arterial cannulation. To continue the plan of care, a second line was placed into a femoral vein and the vascular surgery service was consulted for line removal of the patient’s IJ CVC.
 Click for large image | Figure 1. Anterior posterior chest X-ray depicting the central venous catheter on the left side of the body as indicated by the black arrow. |
During the initial investigation, the IJ CVC was connected to a pressure transducer, which revealed low-pressure venous tracing. Blood samples drawn from the line noted that the blood was bright red (Fig. 2) and an ABG obtained from this sample was largely consistent with suspected arterial blood, with a pH of 7.39, PaCO2 of 30, PaO2 of 242, and HCO3 of 17.2. Given these results, a computed tomography (CT) scan was ordered and confirmed that the CVC was in fact not in the aorta, but rather that the patient had an anomalous pulmonary venous connection with veins from the left upper lobe draining into the left innominate vein (Figs. 3, 4). Considering this new information, the catheter was removed from the left internal jugular vein without complications. The patient’s hemodynamics improved after goal-directed resuscitation, and he was extubated the following day. The patient was discharged home on hospital day 5.
 Click for large image | Figure 2. Blood drawn from the central venous catheter (CVC). |
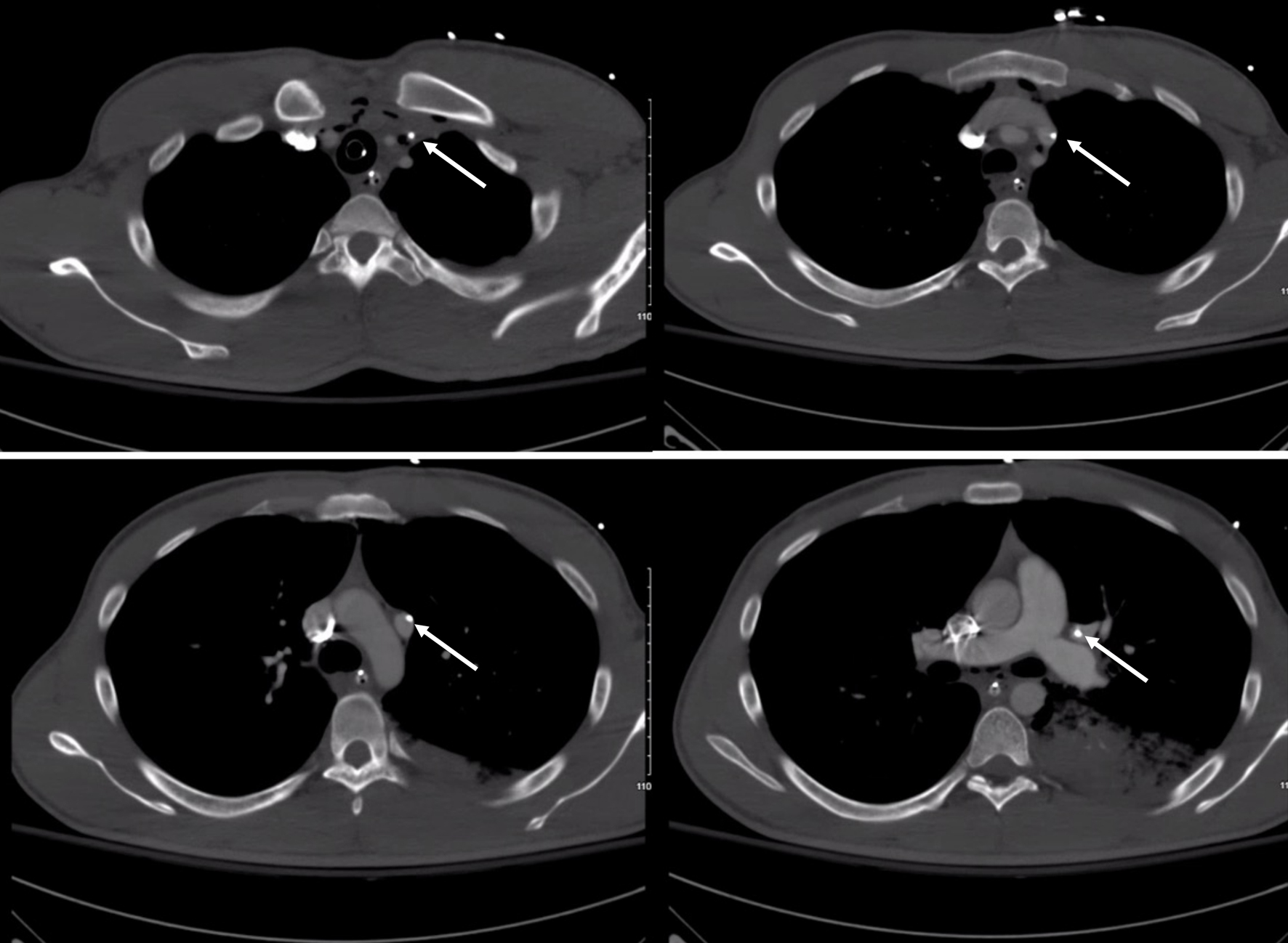 Click for large image | Figure 3. Computed tomography axial images showing the course of the central line catheter. The white arrow shows the tip of the central line catheter in each image. |
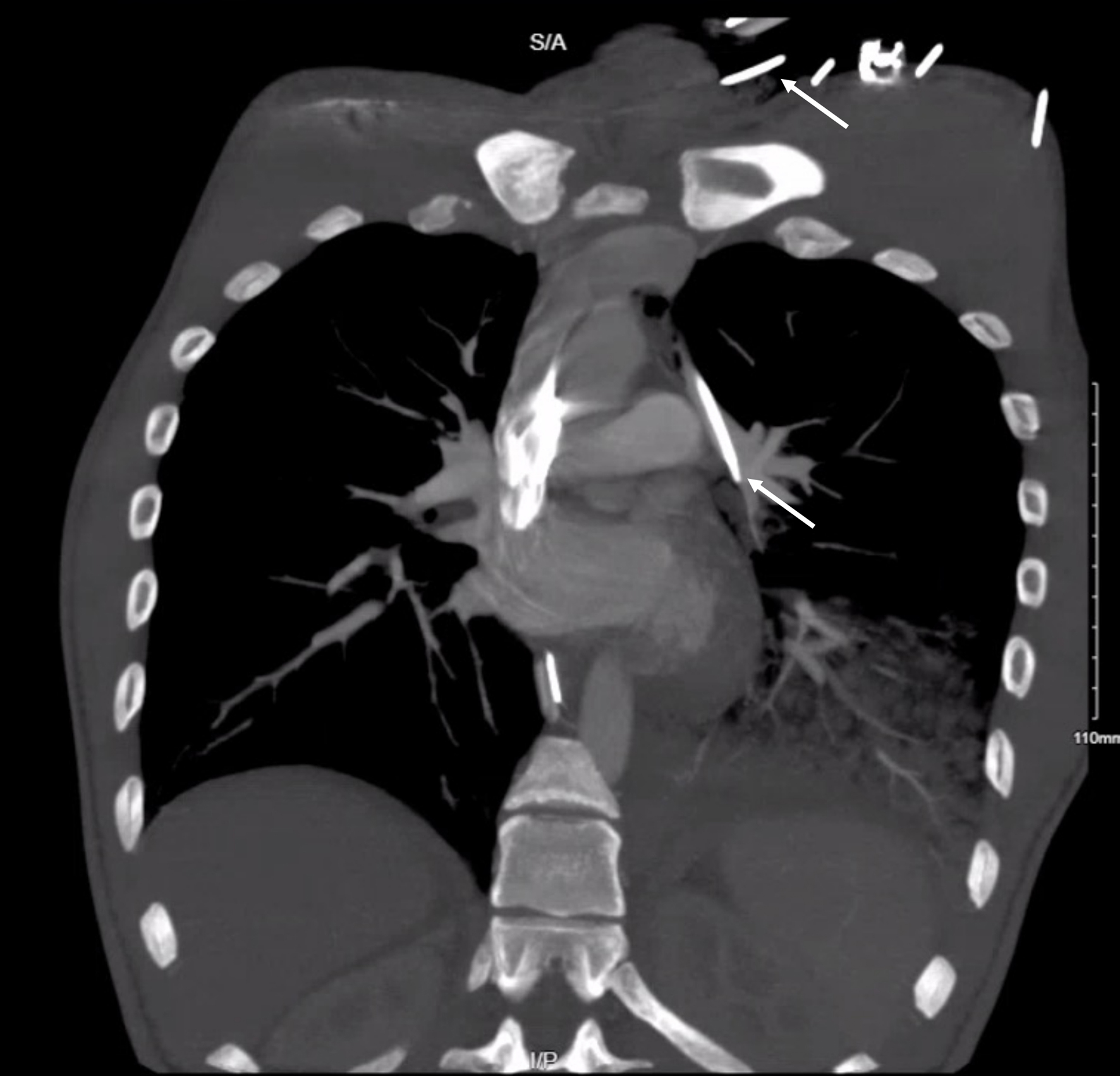 Click for large image | Figure 4. Computed tomography coronal image showing the course of the central line catheter on the left chest. The white arrow shows the tip of the central line catheter. |
| Discussion | ▴Top |
PAPVR is a rare congenital anomaly in which one or more pulmonary veins drain into the right atrium or its tributaries instead of the left atrium. While right-sided PAPVR is more common, left-sided variants are quite rare, accounting for less than 10% of all PAPVR cases [4]. The clinical significance of these anatomic variants depends on several factors, including the number of anomalous veins involved, site of drainage, and presence of associated cardiac anomalies. Isolated left-sided PAPVR without other cardiovascular abnormalities is typically asymptomatic and may remain undetected for years [3]. However, in some cases, it can lead to right heart volume overload, predisposing the patient to conditions such as atrial fibrillation, pulmonary hypertension, and paradoxical embolism. The management of PAPVR depends on clinical presentation and associated complications. Asymptomatic patients may require no specific intervention other than regular follow-up to monitor for any changes in cardiac function [2].
PAPVR is primarily diagnosed through imaging modalities such as echocardiography, computed tomography angiography (CTA), or magnetic resonance imaging (MRI). In this case, the anomaly was confirmed by contrast-enhanced CT, which provided detailed anatomical information about the anomalous venous drainage. One drawback of echocardiography in diagnosing PAPVR is its reliance on user skill, which necessitates substantial practice to achieve proficiency [4]. In a retrospective study conducted by Wong et al, the accuracy of routine echocardiography in detecting PAPVR was evaluated. Fifty patients with PAPVR were identified, and their routine echocardiographic reports were reviewed and compared with findings from surgery or catheterization. Confirmation of PAPVR was available for 45 patients, and the diagnosis was missed in 15 of those cases - a staggering 33% miss rate. Notably, the use of color flow mapping improved detection of PAPVR, with a 7% missed diagnosis with, versus 62% without the use of color flow [5].
Multidetector CT (MDCT) angiography is a popular imaging modality used for identifying anomalies in pulmonary venous drainage owing to its non-invasive approach, rapid scanning process, and ability to produce detailed multiplanar images [6]. In a retrospective study by Ho and colleagues, they sought to determine the lobar distribution and associated radiologic and clinical findings of PAPVR in the adult population using MDCT [7]. Forty-seven cases of PAPVR were identified from 45,538 cases of contrast-enhanced CT examinations and were confirmed by two cardiothoracic radiologists. The prevalence of PAPVR was noted to be 0.1%, with an average patient age of 58 years and female predominance of 58%. Of note, the investigators reported that other imaging techniques and reported cardiopulmonary symptoms lack both sensitivity and specificity for diagnosing PAPVR, emphasizing the critical role of MDCT in accurate diagnosis [7].
MRI can also be used in diagnosis and assessment of PAPVR [7]. MRI provides detailed images of the heart and surrounding structures, allowing visualization of the exact location and extent of the anomalous venous connections. One drawback that may be associated with MRI scans is their relatively higher cost and ease of access compared to that of CT scans. However, it can negate the need for CTA and is an important supplement to inadequate echocardiography [7].
In patients who are symptomatic, primary repair of PAPVR should be evaluated [5, 8]. Surgical guidelines currently suggest considering surgery if the shunt fraction (Qp/Qs) exceeds 1.5, regardless of whether or not symptoms are present [8]. The shunt fraction is the ratio of total pulmonary blood flow to total systemic blood flow. A normal Qp/Qs ratio of 1:1 indicates no shunting. If the ratio is greater than 1, the shunt is most likely from the systemic to the pulmonary circulation, whereas if the ratio is less than 1, the shunt is most likely originated from the pulmonary to the systemic circulation [8].
Surgical treatment of PAPVR is associated with excellent clinical outcomes. In a 2007 study by Alsoufi et al, clinical and echocardiographic follow-up was obtained for 306 patients who underwent surgery for PAPVR [8]. Among the 306 patients, 236 (77%) were children with a median age of 5.3 years [8, 9]. There were no reports of early or late mortality. At 15 years, the rates of freedom from reoperation, vena cava obstruction, pulmonary vein obstruction, and pacemaker implantation were 97%, 97.8%, 86%, and 99.1%, respectively. Various preoperative and intraoperative factors may contribute to improved long-term outcomes and lower morbidity and mortality rates. The type of suture used for the anastomosis, the technique in which the anastomosis is performed, the expertise and comfort of the surgeon, the amount of time on cardiopulmonary bypass, and the aortic-cross clamp cross time are some of the various factors that can contribute to more successful outcomes in individuals requiring treatment [10]. This emphasizes the importance of understanding the pathological anatomy and the specific pathophysiology in suspected cases.
In the United States, over 5 million CVCs are placed annually [11]. The use of ultrasound guidance has lowered the incidence of mispositioned CVCs entering neighboring vessels/organs from 8.4% to 1-3% [12]. In this case, the CVC was not placed into the aorta, despite radiography indicating the CVC in the left thoracic cavity. However, it is crucial for providers to be familiar with prevention and management of this complication. The usual method to help clarify if arterial puncture has occurred is to check the color and pulsation of the blood at the needle hub before inserting the guidewire and catheter [13]. However, ultrasound guidance and pressure are considered more reliable options [14]. The management of arterial cannulation is challenging and can differ with respect to the artery that is accessed, emphasizing the importance of clinical judgment [13]. While manual pressure may be adequate for small-caliber CVCs in accessible vessels, it is inadequate for deeper vessels and larger-caliber CVCs. Percutaneous arterial closure devices, which employ bioabsorbable plugs and suture-mediated closure techniques, have proven to be effective treatment options for subclavian and femoral arteries [11]. However, their applicability is limited by the vessel depth and patient-specific anatomy. Surgical exploration may also be performed if the site is accessible. Otherwise, endovascular therapy should be considered as first-line if performed timely with the proper resources in a hospital. Fortunately, this patient did not need further surgical or endovascular intervention upon confirmation of the CVC tract.
Learning points
Left-sided PAPVR is a rare anomaly that can be incidentally discovered during routine imaging, as demonstrated in our case, following post-central line insertion imaging. While often asymptomatic, it can lead to significant hemodynamic consequences and necessitate careful evaluation and management. This case emphasizes the importance of identifying clinically relevant anomalies to ensure optimized patient outcomes in clinical decision-making and raise awareness of anatomical variants involved with common hospital procedures.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the patient. Ethical approval was obtained from our institutional review board (IRB #23-46).
Author Contributions
Chris Andraos, Sean Hormozian, Aldin Malkoc, Nia Robinson, and Keith Glover: conceptualization, methodology, and writing - original draft. Michael Neeki, Samuel Lee, Samuel Schwartz, and Keith Glover: writing - review and editing. All authors read and approved the final manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available in the article.
Abbreviations
CVC: central venous catheter; IVC: inferior vena cava; PAPVR: partial anomalous pulmonary venous return; SVC: superior vena cava
| References | ▴Top |
- Sundjaja JH, Bordoni B. Anatomy, thorax, lung veins. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024.
pubmed pmc - Singhal K, Newton AD, Corbett C, Predina JD. Management of partial anomalous pulmonary venous connections in patients requiring pulmonary resection: a case report and systematic review. J Thorac Dis. 2017;9(12):5434-5439.
doi pubmed pmc - Konduri A, Aggarwal S. Partial and total anomalous pulmonary venous connection. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024.
pubmed - Javangula K, Cole J, Cross M, Kay PH. An unusual manifestation of left partial anomalous pulmonary venous connection. Interact Cardiovasc Thorac Surg. 2010;11(6):846-847.
doi pubmed - Wong ML, McCrindle BW, Mota C, Smallhorn JF. Echocardiographic evaluation of partial anomalous pulmonary venous drainage. J Am Coll Cardiol. 1995;26(2):503-507.
doi pubmed - Pandey NN, Sharma A, Jagia P. Imaging of anomalous pulmonary venous connections by multidetector CT angiography using third-generation dual source CT scanner. Br J Radiol. 2018;91(1092):20180298.
doi pubmed pmc - Ho ML, Bhalla S, Bierhals A, Gutierrez F. MDCT of partial anomalous pulmonary venous return (PAPVR) in adults. J Thorac Imaging. 2009;24(2):89-95.
doi pubmed - Alsoufi B, Cai S, Van Arsdell GS, Williams WG, Caldarone CA, Coles JG. Outcomes after surgical treatment of children with partial anomalous pulmonary venous connection. Ann Thorac Surg. 2007;84(6):2020-2026.discussion 2020-2026.
doi pubmed - Karamlou T, Gurofsky R, Al Sukhni E, Coles JG, Williams WG, Caldarone CA, Van Arsdell GS, et al. Factors associated with mortality and reoperation in 377 children with total anomalous pulmonary venous connection. Circulation. 2007;115(12):1591-1598.
doi pubmed - Discalzi A, Maglia C, Ciferri F, Mancini A, Gibello L, Calandri M, Varetto G, et al. Percutaneous closure of accidentally subclavian artery catheterization: time to change first line approach? CVIR Endovasc. 2022;5(1):23.
doi pubmed pmc - Bhutta ST, Culp WC. Evaluation and management of central venous access complications. Tech Vasc Interv Radiol. 2011;14(4):217-224.
doi pubmed - Ezaru CS, Mangione MP, Oravitz TM, Ibinson JW, Bjerke RJ. Eliminating arterial injury during central venous catheterization using manometry. Anesth Analg. 2009;109(1):130-134.
doi pubmed - Leijdekkers VJ, Go HL, Legemate DA, Reekers JA. The use of a percutaneous closure device for closure of an accidental puncture of the aortic arch; a simple solution for a difficult problem. Eur J Vasc Endovasc Surg. 2006;32(1):94-96.
doi pubmed - Sallam M, Al-Hadi H, Rathinasekar S, Chandy S. Comparative study of the radial and femoral artery approaches for diagnostic coronary angiography. Sultan Qaboos Univ Med J. 2009;9(3):272-278.
pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.